 What in the world is a zwitterion? What is its importance?
What in the world is a zwitterion? What is its importance?
Molecules are bonded atoms that act as a unit. They are electrically balanced. This means they contain a total number of electrons equal to the number of protons. If a chemical structure does not possess an equal number of electrons and protons it is an ion.
Molecules as a unit are electrically neutral. However, there may be local charges due to geometry or electronegativity. These charges are mostly less than that of a proton or electron. There is an exception. It is the zwitterion. This kind of ion has a full positive and a full negative charge.
In organic chemistry there may be an alkyl backbone consisting of carbon (C) and hydrogen (H). Other groups (pendant groups) include hydroxyl, amino, and carboxylic groups (-OH, -NH2, and -COOH). It is the chemistry of the pendant groups that leads so to the formation of the zwitterion.
Example Formation of a Zwitterion
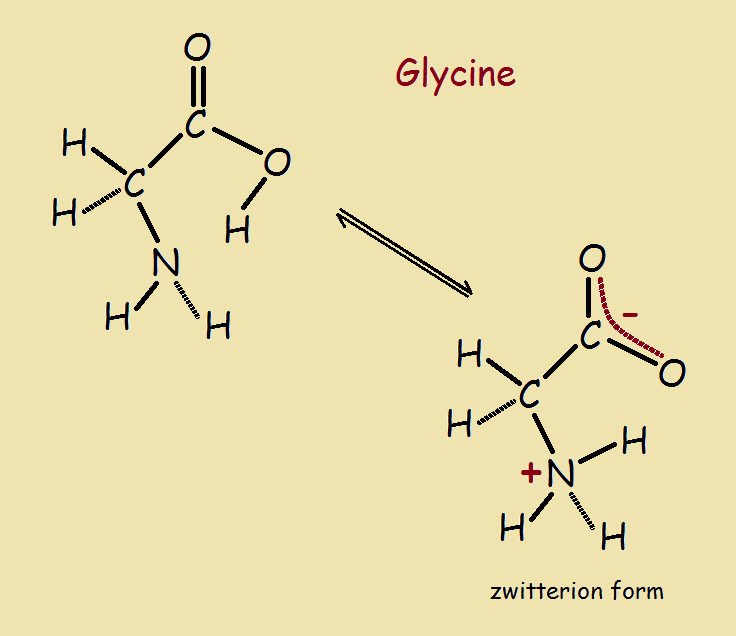
One easy example of how a near neutral molecule becomes a zwitterion is glycine (NH2-CH2-COOH). Note the amino group (-NH2), the carboxylic group (-COOH), and the simple carbon and hydrogen alkyl skeleton, a single methylene group (-CH2–) group. This molecule does not exist in straight line form. The bonds are at angles. In addition, the molecules are dynamic. They are able to twist and turn.
The image shows how the molecule eases transfer of a hydrogen atom resulting in the formation of a zwitterion.1 Written out, the zwitterion is +NH3-CH2-COO⁻¹. Thus a full positive charge, equal to that of a proton, is present at the left end of the molecule. A full negative charge, equal to that of an electron, is present at the right.
The overall charge is zero. The zwitterion has the distinction of being both a double ion and a molecule.
Properties and Applications of the Zwitterion
The charge of zwitterions increases solubility in polar solvents such as water. Since amino acids and some other compounds form zwitterions, it seems solubility is important. In addition, the folding of long chain molecules is seriously affected by electrical charge.
An area of current research is zwitterion transport. Other than in biochemistry, research into this form of ion includes in the fields of nanotubes and fullerenes.
1 Glycine forms zwitterions when it is dissolved in water. Water lowers the energy required to form the double ion. For amino acids in general, the energy increase associated with carrying a negative charge is lowered by spreading over the two oxygen atoms (see associated image). Such spreading out is called resonance.
Note: You might also enjoy The Persistent Triphenylmethyl Free Radical
References:

That’s certainly a very unusual substance.