Although most of us are not chemists, we recognize certain chemical terms. Some terms are more familiar than others. We know orange juice, tomatoes, and vinegar are “acid” or acidic. Other terms are less familiar, such as “free radical”. We’ve heard them, and we have a faint idea of what they mean, but we don’t really understand what they are.
Amines, we may realize, are in part responsible for the fishy odor we seek to eliminate when cooking. To the chemist, an amine is an organic compound that includes at least one –NH2 group. N is the symbol representing a nitrogen atom, and H represents hydrogen. The word amine reminds us of the word ammonia, and rightly so. Ammonia is familiar to us as a liquid. But household ammonia is actually a gas dissolved in water. Its chemical formula is NH3.
The name ammine also comes from ammonia. Ammines are very closely related to ammonia. In this instance, the entire molecule, NH3, rather than a group such as NH2, is involved. Let’s examine a typical example of an ammine that is simple, but unfortunately, has been given a rather complex name: tetramminediaquacopper(II).
Ammines: An Example
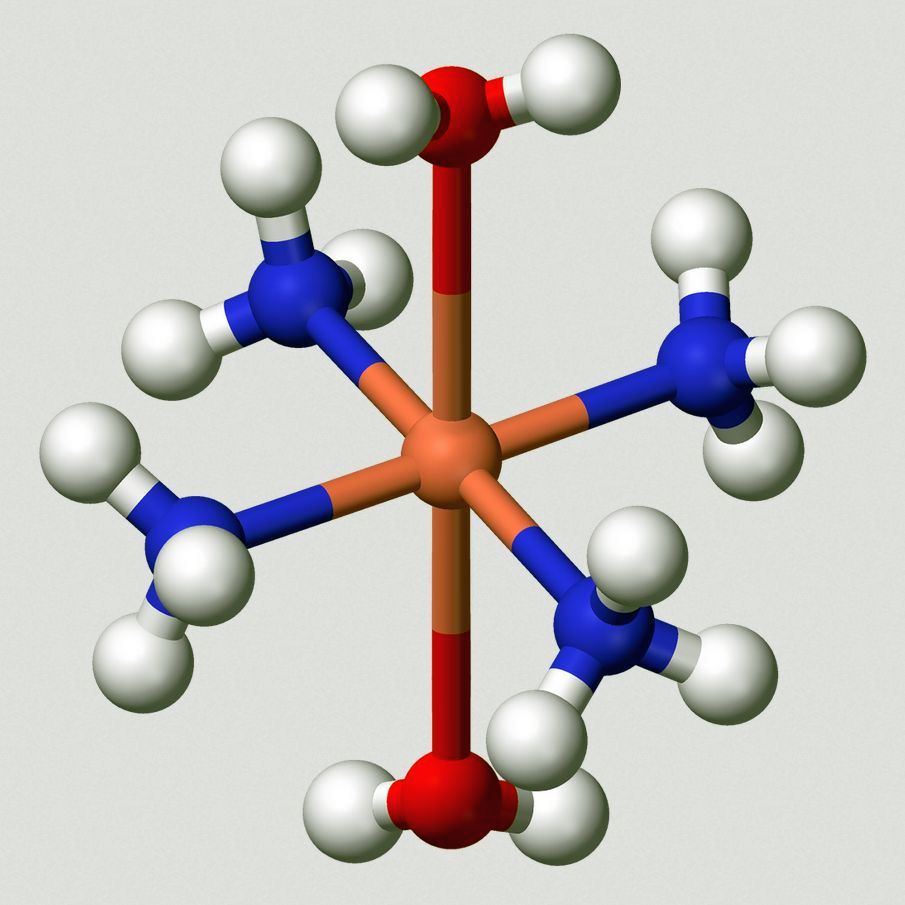
Notice the image… The flesh-colored central portion represents the copper ion Cu+2. This ion is a single atom of copper with two (negative) electrons stripped away, leaving a net charge of +2.
The top and bottom red and white balls represent oxygen and hydrogen, that is, water, H2O.
The other 4 structures, each consisting of one blue and three white balls, represents ammonia. Thus, in shorthand notation, we can write the entire structure as,
[Cu(NH3)4(H2O)2]+2
Ammines and Ions
The electrically neutral ammonia and water molecules combined with the transition metal ion, thus constituting a coordination complex. Why is such a structure stable? Stability comes reducing energy availability. A simple metal ion is a long atom with an unbalanced electrical charge. All the charge centers on the metal.
Now water and ammonia have oxygen and nitrogen atoms, respectively. Such atoms are rich in electron density, which they readily share with the d orbital of the metal ion. In so coordinating with the metal ion, the structure becomes much larger. The ion assumes somewhat the overall shape of an ellipsoid. The resultant spreading of the electric charge increases ion stability.
Ammines and Hydrogen Storage
Certain concepts for improved energy cells call for the “storage” of hydrogen. The ammonia within an ammine transition metal complex is of great interest in this regard. See the references section for one technical article on the subject. Another technical article discusses the use of a cobalt ammine for the production of cobalt nanoparticles. Doubtless other possible applications will be revealed in future.
An Interesting Side Point
I first learned, many years ago, of ammine complexes. The first I became familiar with was the transformation of nickel chloride into hexamminenickel chloride. What particularly caught my attention was the change of color from green to violet. This is due to the change in wavelength of reflected light. Notice the following video which demonstrates what I experienced…
References:
- Chemguide: Naming Complex Metal Ions
- Silicon Tetrachloride Acts Like a Strongly Electronegative Atom
- The Journal of Materials Chemistry: Metal ammine complexes for hydrogen storage
- Journal of Coordination Chemistry: Cobalt(III) ammine complexes as precursors in the synthesis of cobalt nanoparticles
← Back to Classic Science
← Home