
The woman of the house comes out of her garden. Having worked for hours, she has a “powerful” thirst that only a glass of water can quench. She opens the freezer door, clinks a few cubes of ice into her glass, and then turns to the sink and fills the voids between the cubes with pure water from the tap.
Water consists of two hydrogen atoms and one oxygen atom – H2O. At first thought, it would seem there should only be one kind of water. This is not accurate, however. That is so because there are isotopes of both hydrogen and oxygen.
To understand what an isotope is, it is important to realize that the number of protons in the center or nucleus of an atom defines an element such as hydrogen or oxygen. All hydrogen has one proton with its plus charge. Outside the nucleus, to balance that electrical charge, an atom of hydrogen has one electron with its negative charge.
However, the nucleus can also contain a specific number of electrically uncharged neutrons. The neutron has a mass not much different from that of a proton. So the addition of one or more neutrons increases the mass of the atom, without changing the hydrogen atom into another element. It continues to be hydrogen, only it is heavier.
Thus, there are actually 18 stable different kinds of water. The explanation is simple.
Hydrogen Isotopes
Most hydrogen atoms contain no neutrons. A form of hydrogen that has an atomic mass of 1 is called protium (1H). Hydrogen with one proton and one neutron, having an atomic mass of 2, is called deuterium (2H). Tritium (3H) possesses two neutrons per nucleus. Tritium is radioactive. However, tritium’s half-life is about 12.3 years. So it is considered relatively stable.
What is in a name? An oxygen isotope by any other name would provide water as sweet… In fact, although the isotopes of hydrogen are of unusual significance, warranting the use of special names for each of the three varieties of hydrogen atom, oxygen and most other atomic isotopes are not given unique names. They may be listed by the element name plus the number of nucleons (protons and neutrons) it has, such as Cobalt-60, Carbon-14, and so forth.
Oxygen Isotopes
As is true for hydrogen, oxygen occurs as an assortment of isotopes. However, only three of these isotopes are considered to be stable: 16O, 17O, and 18O. Other radioisotopes of oxygen are quite short-lived.
Water Isotopes: “Kinds” of Water
At first it might seem that kinds of water with the chemical structure H2O based on isotope differences only, are 9 in number:
1H216O,
1H217O, 2H217O, 3H217O,
1H218O, 2H218O, and 3H218O
However, there are actually 18 differing isotope kinds of water. This is because each of the two hydrogen atoms in a particular molecule can be a different isotope, giving these 9 additional varieties of water:
1H2H16O, 1H3H16O, 2H3H16O,
1H2H17O, 1H3H17O, 2H3H17O,
1H2H18O, 1H3H18O, and 2H3H18O
Water Composition Percentages
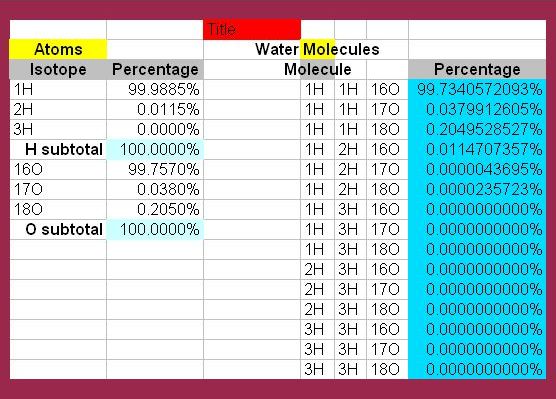
Of course, an ordinary glass of water from the tap does not contain equal amounts of the 18 stable kinds of water. The percentages of each vary considerably. Protium makes up about 99.9885% of all hydrogen. Deuterium makes up 0.1150%. Tritium is so scarce (which is good, since it is highly radioactive) it can be discounted. If we remove it from consideration altogether, this means there are now only 6 kinds of water to consider.
The most prevalent form of oxygen in nature is 16O, at 99.7570%. 17O constitutes a mere 0.0380% of the total. 18O represents 0.2050%. This leads to a chart of percentages of each form of water found in nature. Tritium hydrogen is so inconsequential, it is listed at 0.0000 percent.
The work of mathematical conversion was accomplished by mathematician Mike DeHaan, for whose efforts I am grateful. In fact, the image below is derived from his enumeration spreadsheet.
Water Isotopes in an 8-Ounce Glass
An 8-ounce glass of water taken from the average tap contains the following quantities of 6 kinds of water in order of descending quantity:
7.9787 fl.oz. of 1H216O
0.0164 fl.oz. of 1H218O (1 drop)
0.0030 fl.oz. of 1H217O (1/6th of 1 drop)
0.0008 fl.oz. of 1H2H16O (1/25th of 1 drop)
Less than 1/1,000,000 fl.oz. of 1H2H18O (negligible)
About 1/30,000,000,000 fl.oz. of 1H2H17O (negligible)
The Drink That Refreshes However Many Water Isotopes It Contains
As much as many people may like to think another beverage better accomplishes the purpose, water is the drink that refreshes. Only water, as I’ve shown here, is not quite as simple as most of us suspected. Its six water isotopes make it six drinks in one.
Note: You might also enjoy Polymeric Water Clusters

Thanks a lot! I’ve received detailed answers to a lot of the questions I started to ask myself over the years.
My interest is learning how the water on planet Earth has the unusual characteristic of becoming more dense as the temperature drops, BUT, just before the freezing point that curve reverses itself and the water becomes LESS dense! Know of any other compound that has THAT characteristic … or is only Earth blessed with this extremely unusual “water”?
As water freezes, the shape of the molecule becomes a hexagon due to polar electrical charges of the water molecules. The hexagon’s volume is larger than the same amount of water molecules would be in the liquid state. So the same amount of water molecules are lighter per unit volume when they are frozen as when they are liquid. Ice thus floats. Here is an article that explains the science simply: https://www.scienceabc.com/pure-sciences/why-does-water-expand-when-it-freezes.html and here is a video that discusses water’s behavior https://www.jw.org/en/library/videos/#en/mediaitems/VODBibleCreation/pub-ivwc_3_VIDEO. I hope this helps.