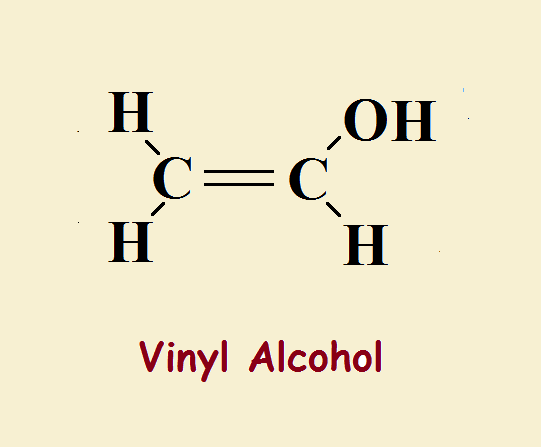
The simplest alcohols contain only carbon, oxygen, and hydrogen. Whereas water contains a hydrogen atom attached to a hydroxyl group, a simple alcohol consists of a chain of carbon and hydrogen atoms attached to a hydroxyl group. Vinyl alcohol is one of the simplest. In fact, it differs by just two hydrogen atoms from ordinary ethyl alcohol.
Ethyl alcohol or ethanol is written, CH₃CH₂–OH. Vinyl alcohol or ethenol is CH₂=CH–OH. Though very similar, ethyl alcohol exhibits very ordinary, straightforward behavior, typical of an alcohol. Ethenol, however, due to its double bond, behaves differently.
Tautomerization
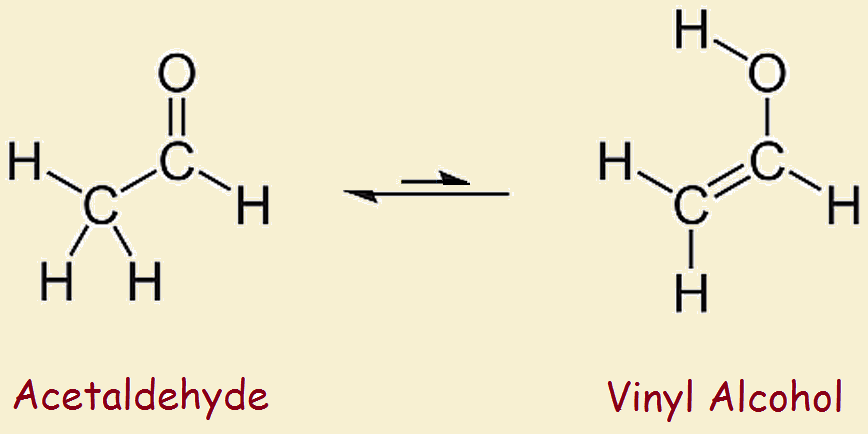
Vinyl alcohol rearranges slightly under ordinary conditions. It tautomerizes to the more stable species acetaldehyde. This variety of tautomerization is termed keto-enol tautomerism. The keto part refers to the carbonyl group typical of ketones and aldehydes, –C=O. The enol part refers to the two functionalities, -ene and -ol, or a double bond and a hydroxyl.
Vinyl Alcohol and Acetaldehyde
The difference in stability between vinyl alcohol and its tautomer, acetaldehyde is considerable; it amounts to more than 42 kilojoules per mole. Thus one does not approach the reagent shelf to pull down a bottle of ethenol. However, theoretical chemistry and simulation studies suggest this alcohol and other enol alcohols play a role in upper atmosphere organic acid synthesis.
Organic Acids and Sea Salt
Such organic acids appear to deplete lofted sea salt particles of some of their chloride, liberating hydrogen chloride into the atmosphere and forming the corresponding organic acid salts.
Undoubtedly an increase in these substances is due to human activity. These, in turn, can affect the way our atmosphere radiates energy. Organic acid salts can also modify cloud nucleation. These two effects, however minimal, may contribute to global climate change.
Note: You might also enjoy Polymeric Water Clusters
References:
- ACS Publications: Atmospheric Chemistry of Enols… 2014
- Wiley Online Library: Research Spotlight Eos, Vol. 93, No. 40 (2012) page 400
- Atmos. Chem. Phys., 14, 11409-11425, 2014: The effect of low solubility organic acids…