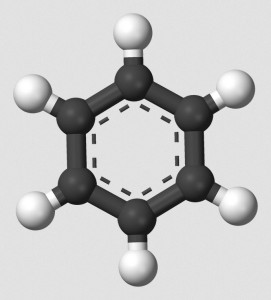
I am a retired chemist. I have no laboratory, and I have no close affiliations, but I do retain a brain. One of my favorite topics is aromaticity. Organic chemistry, essentially the study of carbon chemistry, features aliphatic compounds and aromatic compounds. Aromatic compounds, even being constructed from alternating (conjugated) double bonds, possess resistance against undergoing addition reactions, tending toward substitution reactions instead.
An Idea Begins to Take Shape
Realizing this, I began to wonder about a kind of aromaticity that might utilize triple bonds, rather than double bonds. However carbon-carbon triple bonds force other atoms to take positions along the same axis as the triple bond. A ring created from alternating single and triple carbon-carbon bonds would assume a straight line, the ends of which could not close to form a ring!
The Idea Changes Direction
Is there an element that can form triple bonds with a remaining capacity to form at least two more single bonds that can assume an angle allowing ring formation of with the possibility of the ring possessing a kind of aromaticity? Even if the element can’t bond to itself, can it bond with another element, alternatingly, allowing such a ring to form? And if so, might there be additional varieties of aromaticity?
Is There an Inorganic Chemist in the Audience?
The idea stops there, for me. I recognize my limitations. But I appeal to anyone (unlikely as that is) who may be reading this piece, say an inorganic chemist, to let us know if this concept is totally ridiculous, or if there is a reason to believe it could be possible? Any ideas what elements? Say perhaps a transition element? Clearly a valence of 5 is required, or so I suppose. Thank you.
Note: You might also enjoy Hückel’s Smallest: The Aromatic Cyclopropenyl Cation
References: