 Hair: men bemoan its loss and women fret over its appearance. For decades during the 20th Century, women were enamored by the so-called permanent wave. The most common process for assuring permanency of a hairstyle obtained at the beauty parlor involved a chemical process involving thioglycolate. See, Permanent Wave: Chem-mystery of Curl.
Hair: men bemoan its loss and women fret over its appearance. For decades during the 20th Century, women were enamored by the so-called permanent wave. The most common process for assuring permanency of a hairstyle obtained at the beauty parlor involved a chemical process involving thioglycolate. See, Permanent Wave: Chem-mystery of Curl.
By Extension…
Although this process was used, not to remove hair, but to beautify it, by extension, a closely related process has been used to eliminate hair that grows¹ in undesired places. Notice this chemical reaction that occurs when thioglycolate is used to remove hair².
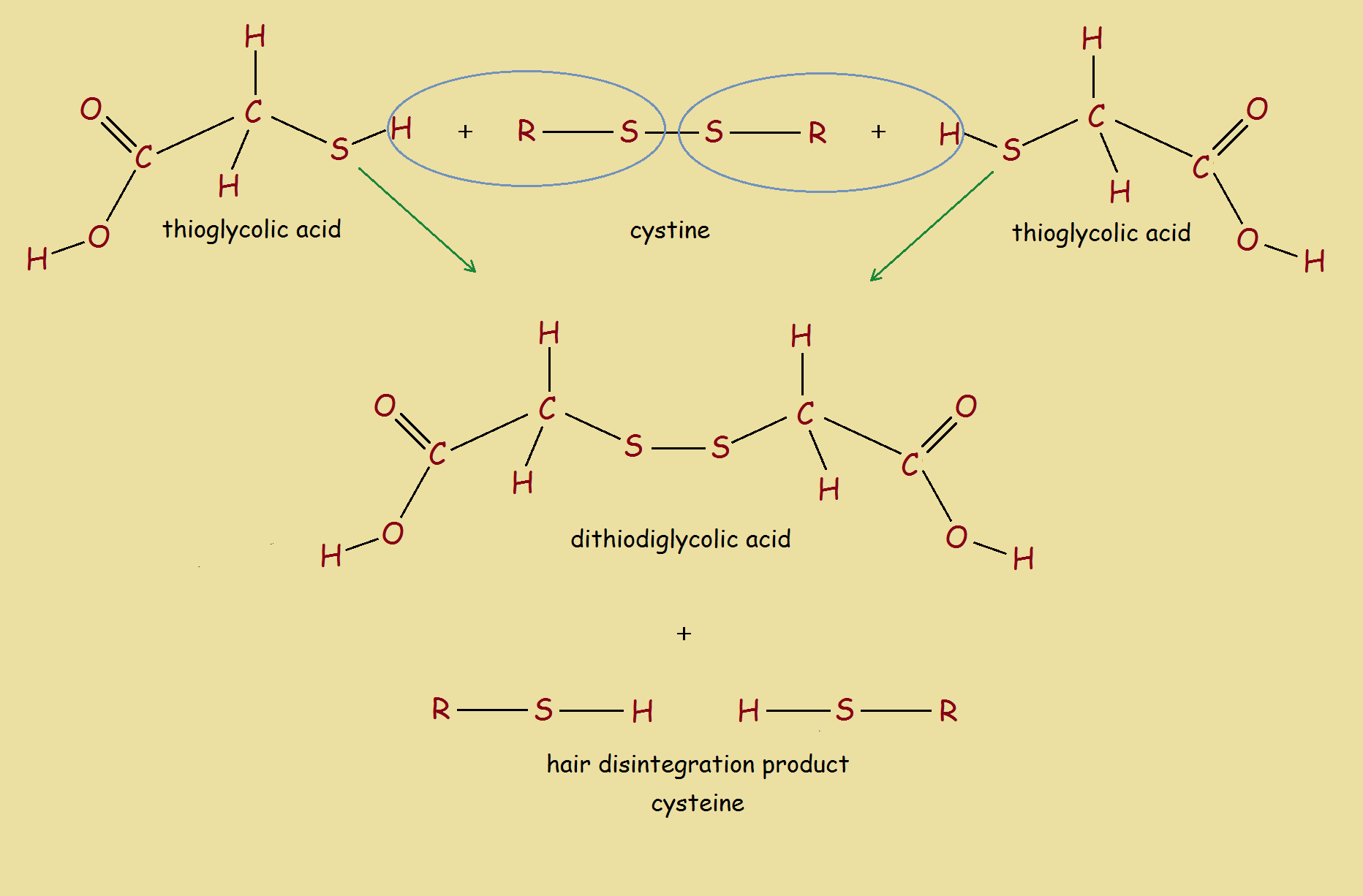
2 HOOC-CH₂-SH + R-S-S-R → HOOC-CH₂-S-S-CH₂-COOH + 2 R-SH
The above reaction reads: two thioglycolic acid molecules plus one cystine (disulfide hair bond) produces two dithioglycolic acid molecules and two cysteine molecules. To better understand the reaction, see the image.
Thioglycolate Hair Remover
It is the breaking of the disulfide bonds in hair protein that promotes the hair disintegration. As footnote 2 tells us, calcium hydroxide or something similar may be present to raise pH. Calcium hydroxide, itself, exhibits depilatory properties.
Hair disintegration in such products takes only a number of minutes, dissolving the hair down into the follicle. Its action is not permanent. Hair begins to grow back shortly after treatment.
1 As to whether hair can actually be said to grow, hair is not alive. Although it is not in the strictest sense entirely correct to say hair grows, neither is it entirely correct to call it waste disposal.
2 In actuality, it is usually calcium thioglycolate, not thioglycolic acid that is used. Also extra calcium in the form of calcium hydroxide may be present, to increase pH.
References:

Think I still would opt for shaving!
[…] early beginning of such treatments, chemicals like thioglycolate were used which broke the structure of hair down. These chemicals were harmful for the hair. […]