Introducing TTBM
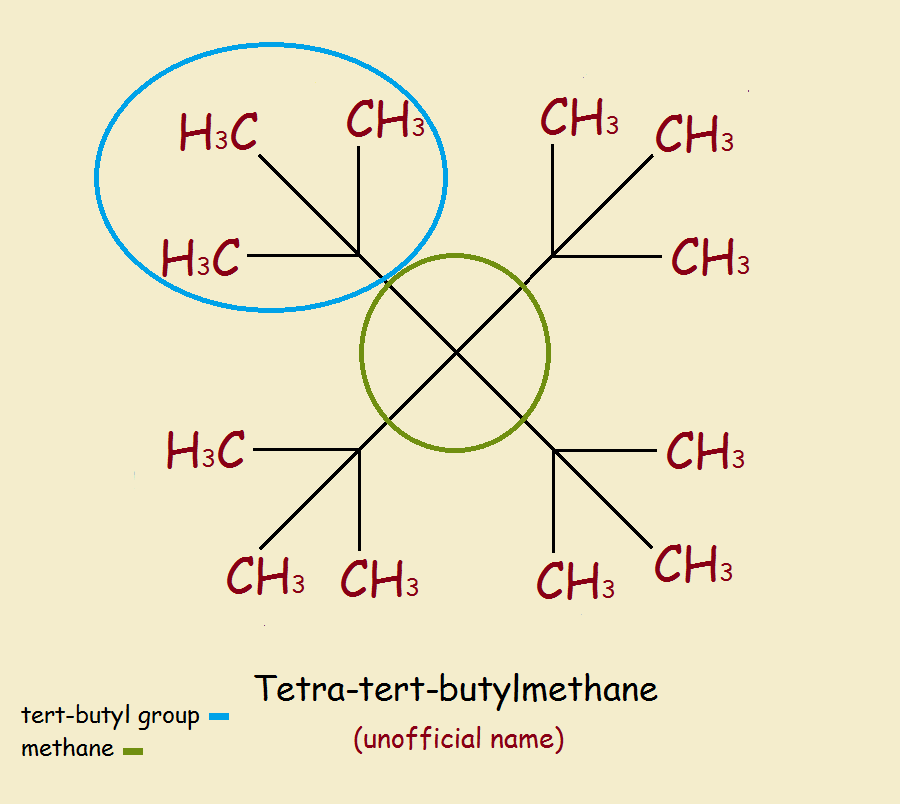
Tetra-tert-butyl methane is the common name of 3,3-ditert-butyl-2,2,4,4-tetramethylpentane.
A glimpse at the structure reveals the overcrowding. Despite the overcrowding, according to energy calculations, the molecule (we’ll call it TTBM) should be capable of existence.
Assembling the Model
Even building the model from its component parts presented a measure of difficulty. The 17 carbon atoms (black spheres) and 36 hydrogen atoms (smaller white spheres) required some effort to assemble into TTBM. Still, it was accomplished with all the component atoms not touching one another. What is the problem or problems?
Synthesis
While attempts are still being made to prepare tetra-tert-butyl methane, it should be obvious that researchers have their work cut out for them. TTBM cannot be made by simple hand-assembly. Reagents are required. Reagent species of necessity are larger than the parts to be assembled.
Reactant molecules and molecular fragments need appropriate spatial orientation. Repulsive forces between already assembled atoms of the developing TTBM structure and additional reactant atoms make the task a most formidable one. It could be likened to assembling an interlocking puzzle while wearing boxing gloves.
Will Tetra-Tert-Butyl Methane Ever Be Synthesized?
Note: You might also enjoy Hückel’s Smallest: The Aromatic Cyclopropenyl Cation
References:
- Journal of Chemical Information and Modeling: What Is the Smallest Saturated Acyclic Alkane that Cannot Be Made?
- The Journal of Physical Chemistry: Structural and Energetics Studies of Tri- and Tetra-tert-butylmethane
- March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure 6th Ed. p232