 The basic formula for sodium metasilicate (water glass) includes two sodium, one silicon, and three oxygen atoms. When it includes no trace of water, the meta-silicate is anhydrous.
The basic formula for sodium metasilicate (water glass) includes two sodium, one silicon, and three oxygen atoms. When it includes no trace of water, the meta-silicate is anhydrous.
Its formula should then be Na2SiO3, right? Well, yes. But, to leave it at that would be less than truthful.
Parts, Just Parts
Sodium meta-silicate can be visualized in two parts. One part is the two sodium ions Na+, each with a +1 charge. The other part is the meta-silicate (SiO3)-2 with its minus two charge.
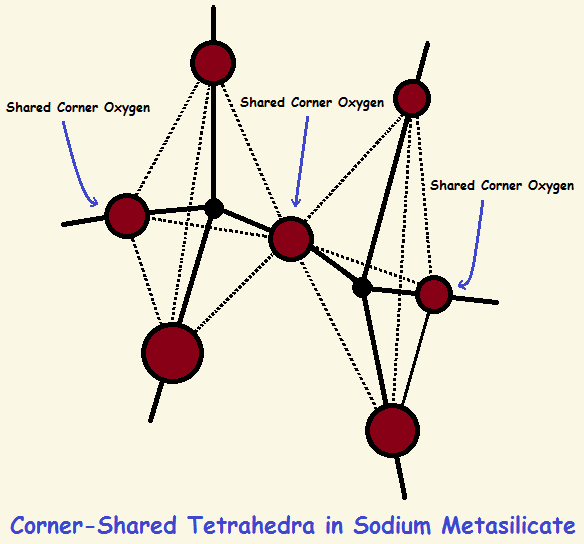
Some atoms can link to form chains. These atoms include carbon, boron, and silicon. Silicon and oxygen atoms of sodium meta-silicate molecules form a polymeric, corner bonded tetrahedra complex. This complex appears as if it has sub-units of SiO4 (silicon atoms central in tetrahedra).
Note the SiO4 structure is apparent only. It is not real. Two of the four oxygen atoms are shared with adjacent silicon atoms on either side. This makes it 1/2 + 1 + 1 + 1/2 = 3 oxygen atoms per silicon, even though it looks to be 4. See the image.
Structure On Dissolution
What is the structure of sodium meta-silicate when dissolved in water? The structure is ionic. Hence the polymer is broken down. The ions are hydrated. The coordination number (the number of water molecules that surround an ion) for sodium is between 4 and 6. The size of the ion plays a major role in the coordination number. It is like fitting a glove on a hand. The larger the hand, the larger the glove.
Negative silicate ions are quite large. They are surrounded by more waters of coordination than sodium ions are. These water molecules “smear” the charge across the entire hydrated cluster. This reduces the energy needed to stabilize the system.
Other Forms of Sodium Metasilicate
Other forms of the meta-silicate, such as the pentahydrate, also form stable tetrahedral substructures. Thus sodium pentahydrate, Na2SiO3•5H2O, forms a polymeric structure SiO2(OH)2-2 with water of hydration. The nonahydrate does so also.
Note: You might also enjoy Cyclosilicates: Beautiful Gemstones of Technological Interest
References:
- Brooklyn College: The Silicon Tetrahedron
- “Structural Inorganic Chemistry” 5th edition, by AF Wells, Oxford Science Publications 1984.
← Back to Classic Science
← Home

It is great.