Carbon is one of the few elements that readily bonds to itself, thus allowing the formation of macromolecular structures. It is not at all an uncommon thing to see a lengthy carbon-based structure, possessing molecular weight well up into the tens of thousands. Compare this with an “ordinary” molecule such as table salt, sodium chloride (NaCl) with its molecular weight—a mere 58.
Drawing Single Bonded Organic Structures
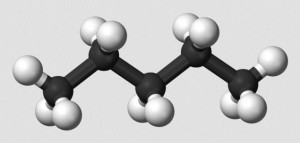
Ordinarily, carbon compounds are written by chemists in as simple a form as possible. Thus, drawing single bond organic hydrocarbons such as n-hexane, we write,
C₆H₁₄
or, a little more in detail,
CH₃(CH₂)₄CH₃
or, perhaps even,
CH₃‒CH₂‒CH₂‒CH₂‒CH₂‒CH₃
Need Greater Specificity?
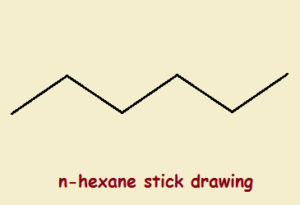
The problem is, carbon single bonds possess tetrahedral symmetry. This means that single bonds between three carbon atoms are not 180o in measure. To draw a simple, non-branched hydrocarbon as a straight line structure is just wrong. A zigzag carbon chain would be an improvement, with angles of about 109o.
Yet another improvement, generally drawn only when specifically needed, is to show any atoms or groups attached to the carbon atoms as above or below the plane of the paper. This acknowledges the 3D nature of molecules. Consider, for example, the strychnine molecular drawing, illustrated below. The hydrogen and nitrogen atoms that are supposedly above the plane of the paper are attached to thin solid black triangles. The hydrogen atoms that are supposedly below the plane of the paper are attached to thin dashed triangles.
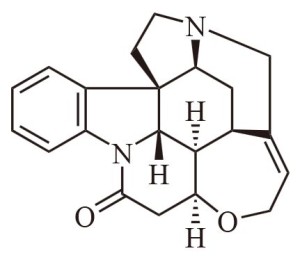
You will note the carbon atoms are shown connected via uniform-thickness, straight-line segments, as if they are all in the plane of the paper. Truthfully, those atoms are not all in the plane of the paper. In actuality, atoms attached exclusively with single bonds are ordinarily quite free to move about. Longer chains, especially, may become considerably twisted.
The solid dark, triangular bonds with their over-sized atoms are drawn in that fashion to illustrate that they are sticking up out of the plane of the paper. The dashed bonds with their undersized atoms are drawn that way to indicate they lie below the plane of the paper. All of this is of value in imparting 3D structure visualization in a 2D medium. This helps chemists avoid errors in his or her evaluations.
Single Bond Organic Hydrocarbons – Additional Comments
There are all manners of additional ways to draw molecules. Whatever accomplishes the task of communication may be used, provided understanding is imparted to the viewer. This primer, it is hoped, is of some help to the reader in drawing single-bonded organic chemical structures.
Note: You might also enjoy The Difference Between Alanes, Alkenes, and Alkynes
References:
← Back to Classic-Science
← Home
