 It is not unusual for school systems to introduce students to chemistry by means of the Periodic Table of the Elements. The table is then broken down into sections: the metals, the non-metals, and the gases.
It is not unusual for school systems to introduce students to chemistry by means of the Periodic Table of the Elements. The table is then broken down into sections: the metals, the non-metals, and the gases.
Before long, the structure of the atom is discussed, including protons, neutrons, electrons, orbitals, shells, and valence. It is the last of these we will briefly discuss here – valence. First a very brief discussion, followed by examples, followed by a puzzling problem (to impart insight).
Valence: A Simple Discussion
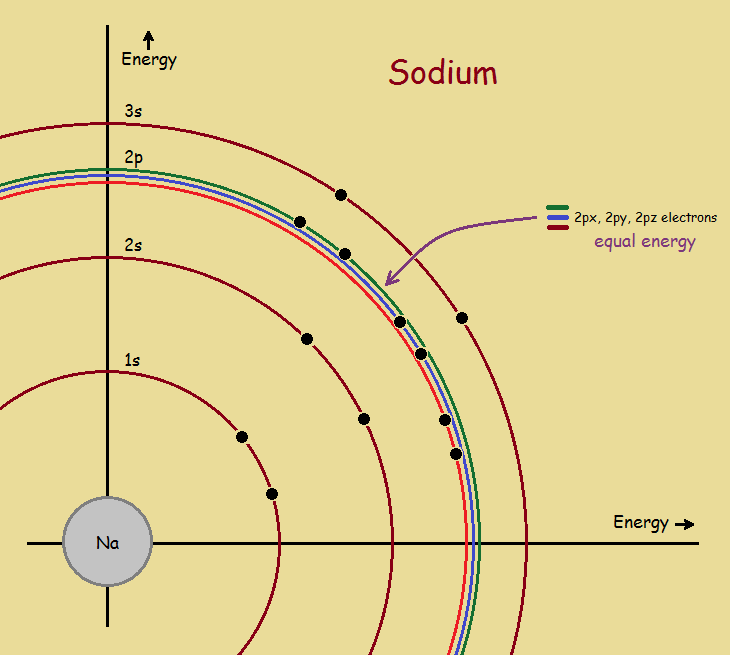
Atoms, although containing positive protons and negative electrons, have a net charge of zero. They are electrically neutral. This means each lone atom has a number of electrons equal to its number of protons. For instance, a sodium atom¹ has 11 protons. It also has 11 electrons. Those electrons are distributed in the following fashion…
1s2 2s2 2p6 3s1
For non-Transition elements, the valence electrons are typically the last listed electrons, in this instance, the 3s1 electron. And sodium’s valence is +1. Why a plus 1? Because if a sodium atom gives up that one electron in chemical reaction, it’s highest remaining shell is completely filled, giving it a configuration similar to the element neon, whose electrons are distributed as…
1s2 2s2 2p6
Such an electron structure is very stable. So much so, that neon is considered an inert gas. This means that neon is chemically unreactive. And so it is, the sodium ion, combined chemically, completely resists the tendency to lose any more electrons!
Another Example
Now let’s consider another example, this time of an atom that is more than willing to gain an electron, chlorine. A chlorine atoms possesses 17 protons and 17 electrons. It’s electron distribution is,
1s2 2s2 2p63s23p5
First, notice the 3p subshell contains one less than the “perfect” number of 6. Chlorine is more than willing to fill that subshell with another electron. When it receives such an electron, its chemical formula is written Cl-1.
Valence Possibilities
Although valence values typically tend toward the lower values of +/- 1 to 4, they can be higher, with one or two metal atoms running as high as +8. Now let us consider a few common examples of compounds and the valences of their constituent atoms.
Compounds and Valences
Example 1:
Nao → Na+1 + e–
Clo + e– → Cl-1
Na+1 + Cl-1 → NaCl [neutral]
———————————————————————-
Example 2:
4 Alo → 4 Al+3 + 12 e–
3 Oo + 6 e– → 6 O-2
2 Al+3 + 3 O-2 → Al2O3 [neutral]
———————————————————————-
Now Our Simple Problem for a Beginning Chemistry Enthusiast
We wish the reader to do just the reverse for the following molecule. Tell us the valencies for the iron and the oxygen in the chemical compound, below. WARNING: This is not all that simple. Yet, it is a solvable problem, even for the beginner, if he/she is astute…
Fe3O4
The Solution

We have seen that the valence of oxygen is -2. Although we have not discussed multiple valences for the same substance, neither have we dismissed the possibility.
If oxygen is -2 in this instance, four oxygen atoms would indicate an eight electron excess in our compound. Since there are 3 iron atoms in our compound, this would suggest a valence of 8/3 electron per iron atom. This is absurd. It suggests there is something peculiar with the valence of iron.
Indeed, there is. Looking up iron either online or in any beginner’s chemistry book, we learn that iron can exhibit two valences: +2 and +3. These two types are often referred to as ferrous and ferric, although modern terminology (whether better or worse) lists them as Fe(II) and Fe(III), respectively.
Now as we saw, in our consideration of aluminum oxide, Al2O3, if we had one atom of ferric iron, our oxide would be Fe2O3. Subtracting this from Fe3O4 would leave FeO.
There you go! This is the formula of ferrous or Fe(II) oxide. The solution to our difficulty is Fe3O4 is the same thing as FeO•Fe2O3. This is magnetite, commonly called ferrous ferric oxide. IUPAC name: iron(II,III) oxide.
In Conclusion
What, then, can the beginning chemistry student learn from our little problem? A number of things.
1. Seeming inconsistencies should never be too quickly dismissed.
2. Valences must always be resolved into integer values.
3. Compounds can be complexes of atoms of the same variety existing at different valences (AKA oxidation levels).
In reality, many minerals exist in nature, some of which are very difficult to resolve into specific chemical formulae, partly due to multiple valencies.
¹ The chemical formula for a neutral sodium atom is sometimes written Nao to indicate it has zero charge.
Note: You might also enjoy Introduction to Chemistry Subscripts and Superscripts
← Back to Classic Science
← Home

Totally different from the way I learned valencies in school. We had to learn them just as integers and we never got given that tricky little problem you demonstrated. Interesting.