What is Saturation? What is Unsaturation?
Even those scientifically uninclined use the words. Saturation, unsaturation, and polyunsaturation are dietary terms closely associated with health.
Most know they are also chemical terms. But they don’t understand what they mean. This is a shame, since “saturation” can be easily understood by any bright student.
Saturated Hydrocarbon Linkage
Living matter (plant or animal) is made of of organic compounds. These include (but are not limited to) fats, carbohydrates, and proteins. All these substances contain a kind of backbone or chain, of atoms of carbon (C) bonded to atoms of hydrogen (H). Each carbon is capable of bonding with four hydrogens.
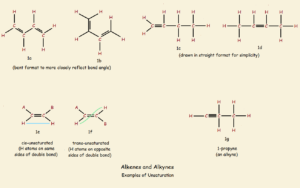
For purposes of illustration, we’ll assume we have before us a chain of four carbon atoms, with every available bond possibility occupied by hydrogen atoms—in other words, n-butane, the “n” standing for a normal, or straight backbone. The structure can be drawn, CH3-CH2-CH2-CH3. The single unsaturation modifications drawn below illustrate what we will discuss here.
CH3-CH2-CH=CH2
CH3-CH=CH-CH3
You will note that these two structures contain the same number of carbon atoms as that of n-butane. Yet they contain two less hydrogen atoms. These chains are not saturated (filled to the limit they can hold) with hydrogen. Later in this article, the layout of the hydrogen atoms is seen to be of great importance, as well.
Geometry of Saturation
The above drawing of the butane molecule is a little misleading. Saturated carbon forms tetrahedral angles of approximately 109.5° not the 180° angles required to form a straight line backbone. It is a little more accurate to draw a zigzag chain, as is illustrated in Figure 1a. The teeth actually assume a slightly obtuse angle.
Unsaturated Alkenes
As was seen above in the case of butane, if two hydrogen atoms are removed from adjacent carbon atoms, the single C-C bond becomes a double C=C bond. The result is called an alkene. And as also noted above, there are two possible locations for the double bond to form. One is between carbon atoms 1 and 2 (fig. 1c); the other is between carbon atoms 2 and 3 (fig. 1d).
There is nothing new if the double bond is drawn between carbon atoms 3 and 4; a mere flip of the molecule shows this is identical to the molecule in fig. 1c.
Unlike the flexibility of the single bond, a double-bond is fairly rigid. It locks the two carbon atoms and their immediately attached four atoms in a plane.
Cis- and Trans- Alkenes
If the double bonded carbon atoms of an alkene is each bonded to another carbon atom, two classes of compound can form: cis-alkenes and trans-alkenes. If, of the four branches attached to the double bonded carbon, two are hydrogen atoms and the other two branches contain carbon, there can exist what are called cis- and trans-isomers of the very same compounds. The difference can be simply illustrated by imagining two carbon-based branches, Branch A and branch B. If such is the case, the compound 1e is cis, and the compound in 1f is trans. The cis-isomer has both branches on the same side of the rigid double bond, whereas the trans-isomer is trans or across the double bond. Generally speaking, cis- chemistry is the chemistry of life.
Alkynes
Remove another two hydrogen atoms from the two carbon atoms of a double bond, and an alkyne is the result (fig. 1g). The carbon atoms of a triple bond, as well as the immediately attached two atoms to those carbons, are locked into a straight-line configuration. Atoms further away are free to rotate. There are no additional isomers such as a cis- or a trans- introduced by the presence of alkyne linkage.
If one of the atoms attached to an alkyne carbon is a hydrogen atom, that atom is said to be acidic—it can often be replaced by a metal atom. One thus needs to be careful what kind of container he puts a liquid alkyne such as acetylene (HC≡CH) into, if he is to avoid chemical attack. The compound resulting from attack of acetylene on metal is an acetylide, some of which are quite explosive.
Polyunsaturated
Monounsaturated – as used in the popular media – means a molecule (for example, peanut oil) that contains only one double bond. Polyunsaturated means each molecule contains a multiple number of double bonds.¹ One example of a polyunsaturated molecule, found in many common vegetable oils, is α-linolenic acid, which contains three conjugated (alternating) double bonds. All of its double bonds take the cis-format. It is categorized as a polyunsaturated, omega-3 fatty acid.
1 Strictly speaking, the term polyunsaturated could refer to the presence of multiple triple bonds, but this is not the intended meaning in commercials.
Note: You might also enjoy Introduction to Chemistry Subscripts and Superscripts
← Back to Classic Science
← Home