
Polymeric water clusters? What are they? And what do they have to do with life?
There’s no point in discussing how important water is to life. It is the single most necessary compound for the existence and survival of human, animal, and plant life.
Why Special?
What makes water special is its unusual behavior. Typical of liquids, as water approaches freezing, its density increases. Yet just before freezing, it suddenly decreases. This is why ice floats. Once it forms, ice insulates the water beneath it. It prevents large bodies of water from freezing solid.
How? Hydrogen bonding. To understand hydrogen bonding, we must first consider the shape of the water molecule.
Geometry of a Water Molecule
A water molecule consists of two hydrogen atoms and an oxygen atom. Oxygen is much larger than hydrogen. This suggests that a molecule of water looks like three balls lying in a straight line.
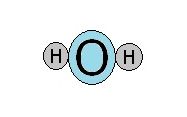
In fact, water is shaped like the head of a mouse.
For this reason, the water molecule affectionately has been called Mickey the Dipole.
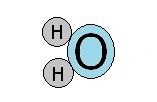
Though the overall charge of a water molecule is zero, there is a small positive charge on one side of the molecule and a small negative charge on the other side. These two poles identify the water molecule as a dipole.
These tiny charges allow weak bonds to form between water molecules. They are hydrogen bonds. These bonds break and form easier than most.
An Illustration
Imagine a vertical cliff with a mountain climber traveling across its face. He attaches and removes ropes to traverse it. He sticks close to the mountain.
It is like that for water molecules. The “ropes” are the hydrogen bonds. However, those move in three dimensions, not two. Hydrogen bonds are also responsible for another characteristic of water. Water forms polymeric clusters.
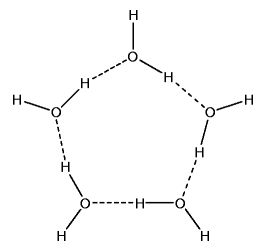
Polymeric Water Clusters
Hydrogen bonds enable the forming of water clusters. These clusters include trimers (3-water clusters). They include tetramers (4-water clusters). They even include pentamers (5-water clusters) and beyond.
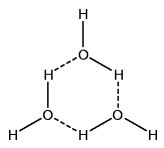
If given the correct conditions, such polymeric water clusters form. And these clusters are structures of increased order. Melting point thus increases. But there is something else, just as important.
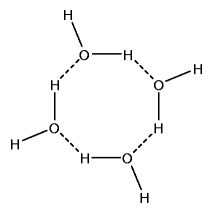
Spaces exist in the centers of polymeric water structures. There is more space per unit volume of ice than there is per unit volume of very cold water. it is this decreased density that causes the ice to float.
This protects the oceans’ aquatic life. Also that of lakes and rivers. Much of earth’s oxygen comes from the oceans.
Is all of this a coincidence? You can judge for yourself. There are just far too many coincidences in favor of the supporting of life for me to believe they are just coincidences.
Finally
Some may cry out, there’s no such thing as polymeric water, at least in any long lasting sense. True, one might envision any number of temporary structures for water. However, the fact that water can and does form polymeric structures with increased interior spaces is evidenced by how as ice freezes it is less dense and floats.
You may also enjoy: Isotopic Varieties of H2O: How Many Kinds of Water are in Your Glass?
References:
- Berkeley University: The Water Trimer
- Cambridge University: Theoretical Study of the Water Tetramer
- Berkeley University: Water Pentamer
