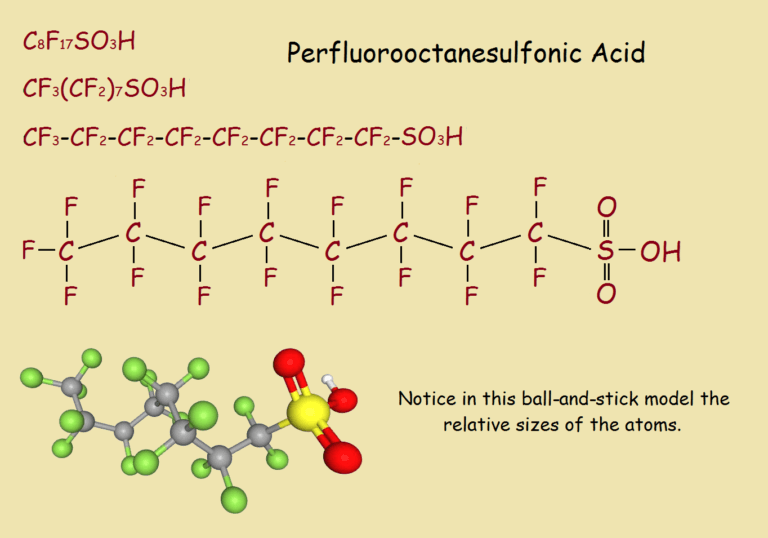
 Perflurooctanesulfonates or PFOs are simple compounds, not found in nature. They are derivatives of perfluorooctanesulfonic acid. Their chemical structure includes atoms of carbon, fluorine, sulfur, and oxygen.
Perflurooctanesulfonates or PFOs are simple compounds, not found in nature. They are derivatives of perfluorooctanesulfonic acid. Their chemical structure includes atoms of carbon, fluorine, sulfur, and oxygen.
Various ways of writing the acid’s formula are seen in the illustration, top to bottom – the simplest to the most complex, followed by a ball-and-stick model.
Commercial Sales
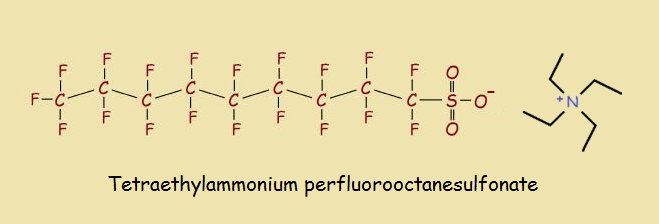
Search for the acid online, and you will find it. It can be purchased as the free acid. However, it is also sold in compound form, such as the tetramethylammonium or tetraethylammonium salts.
Useful?
There’s no question these substances are useful. Consider this… The structure of this acid is not unlike the chemical structure of Teflon®. Teflon is slippery stuff. Teflon, however, is a solid. What if a similar, only soluble, substance could be produced, perhaps even to be sold in a spray can?
Dirt should not stick. It would almost be correct to say a surface sprayed with the stuff would ‘repel dirt’. Ever heard of Scotchgard®?
Compounds of the acid, as mentioned earlier, include cationic (positive ionic) portions such as tetramethylammonium and tetraethylammonium. This reminds us of the cationic portions in many detergents.
Additional PFO and derivative uses include carpet impregnation, waxes, polishes, coatings, and many others… even aircraft hydraulic fluid.
Problematic and Pervasive
Perhaps the main problem with substances related to perfluorooctanesulfonic acid is their ability to gradually dissolve in water. Wastewater experts are concerned that traces of these substances can leach from land-applied biosolids, ultimately reaching, you guessed it, our drinking water.
The sprayed fabric of an item you purchased might eventually reach that glass of icewater your neighbor is sipping. The opposite is just as likely. In fact, the stuff is being found everywhere. Levels are rising, and there is a strong possibility those levels will become critical.
Just a Thought
There are limits on many substances, beyond which, there is concern by governmental agencies for our health. So far, this has not been applied to PFOs or related compounds. That may change in the near future. If so, likely those standards will be enforced.
If so, those standards would doubtless reduce our risk. But, and here’s the thought… suppose we were to receive exposure to every conceivable substance watched by the government at a level just slightly below permissible levels. Would we remain healthy? That is, would the compound effect make us very ill, or worse?
Note: You might also enjoy How to Glue Teflon
References:
- Organization for Economic Cooperation and Development: Hazard Assessment of Perfluorooctane Sulfonate (PFOs) and Its Salts
- Treatment Plant Operator: Wisconsin Agency Asks Wastewater Plants to Test for PFAS
