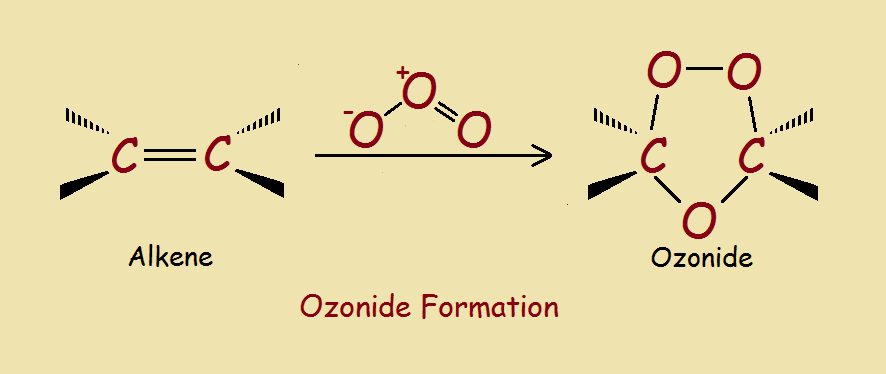
Initially, all three oxygen molecules add to the alkene on the same side of what was previously a double bond. This structure, however, is a transient intermediate, which rearranges to form the ozonide structure. Ozonides are relatively stable. However, they can be readily split to yield a pair of carbonyl compounds.
Organic Ozonides in Synthesis
Some ozonides are explosive, so they are seldom isolated. However, ozonides can be made to undergo a number of useful synthetic reactions. A few of these are illustrated in our third image.
1The organic chemist all too often experiences factors that alter a “typical case”.
Note: You might also enjoy Ozone – the Other Oxygen
References:
- Yale University: Ozonolysis
- Wiley: Angewandte Chemie: Mechanism of Ozonolysis by R. Criegee
- Advanced Organic Chemistry, 4th Ed. by Jerry March – John Wiley & Sons