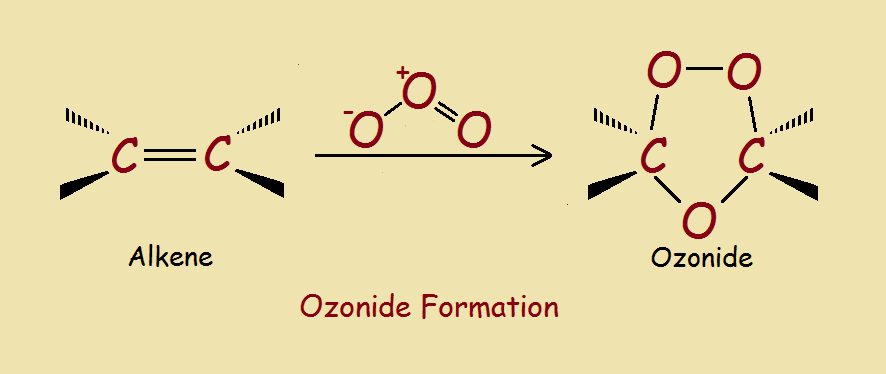
 As you might guess, organic ozonides may be derived from ozone. So let’s first consider the nature of ozone. The ozone molecule, O3 is bent and unstable. It is polarized and is quick to react with unsaturated molecules, notably alkenes and alkynes. The image provided illustrates the result. What, however, is the mechanism producing the result in a typical case?¹
As you might guess, organic ozonides may be derived from ozone. So let’s first consider the nature of ozone. The ozone molecule, O3 is bent and unstable. It is polarized and is quick to react with unsaturated molecules, notably alkenes and alkynes. The image provided illustrates the result. What, however, is the mechanism producing the result in a typical case?¹
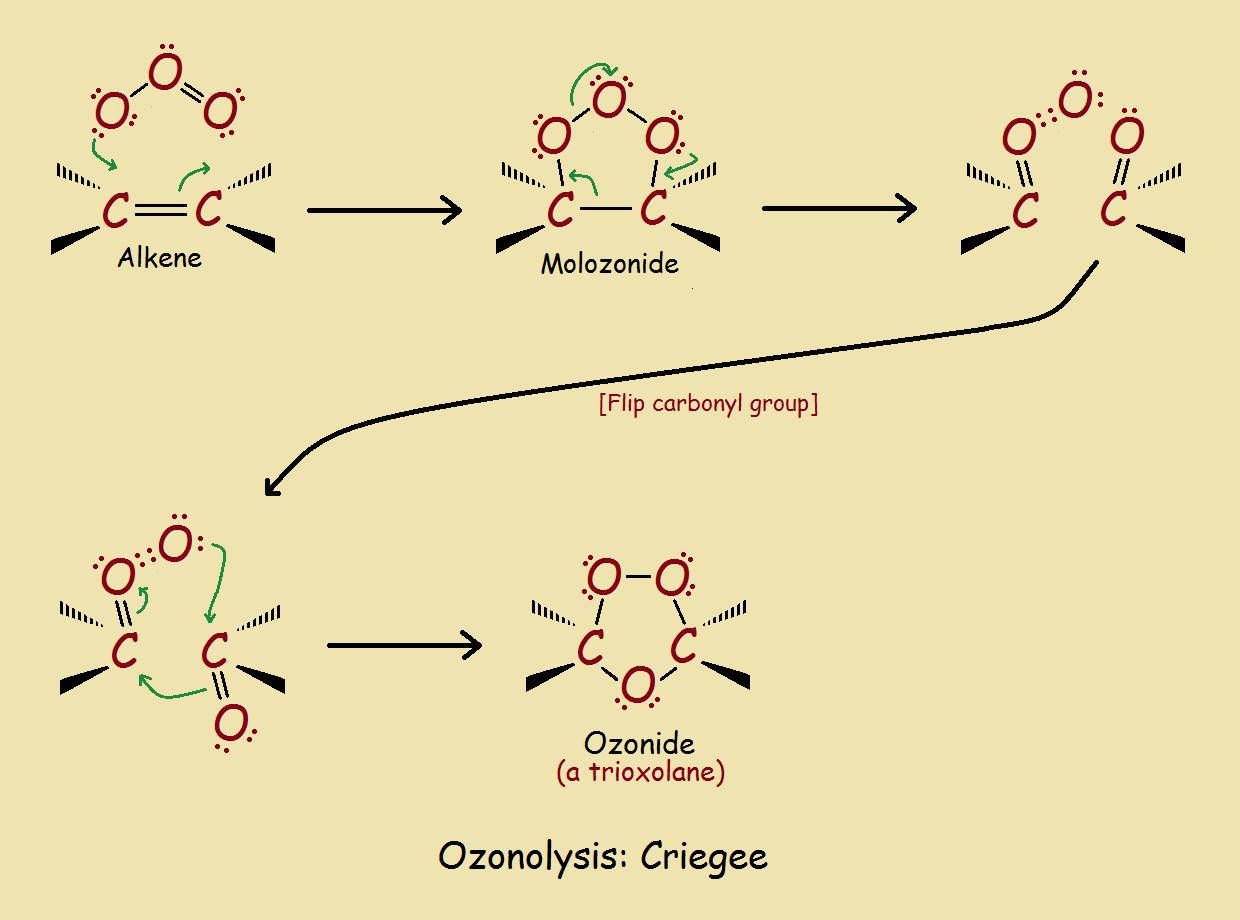
Initially, all three oxygen molecules add to the alkene on the same side of what was previously a double bond. This structure, however, is a transient intermediate, which rearranges to form the ozonide structure. Ozonides are relatively stable. However, they can be readily split to yield a pair of carbonyl compounds.
Organic Ozonides in Synthesis
Some ozonides are explosive, so they are seldom isolated. However, ozonides can be made to undergo a number of useful synthetic reactions. A few of these are illustrated in our third image.
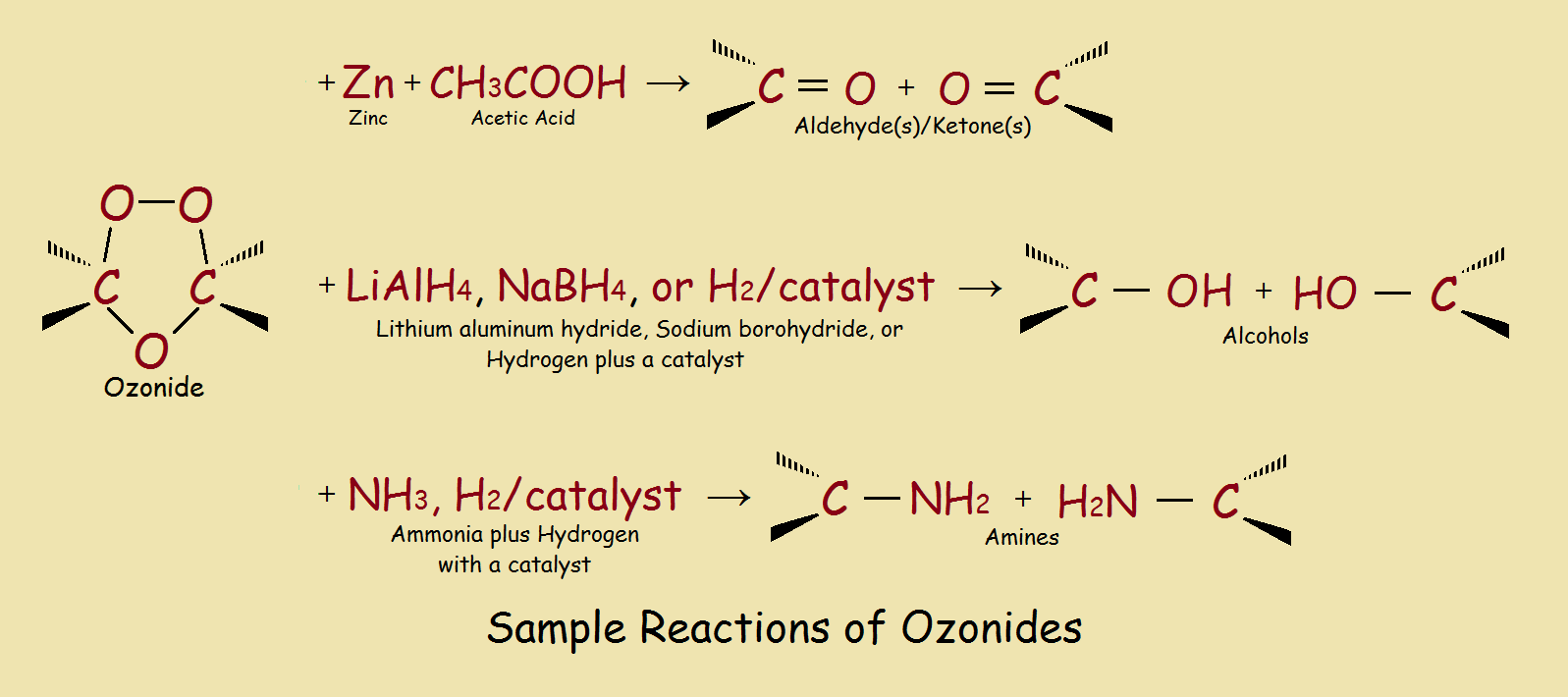
1The organic chemist all too often experiences factors that alter a “typical case”.
Note: You might also enjoy Ozone – the Other Oxygen
References:
- Yale University: Ozonolysis
- Wiley: Angewandte Chemie: Mechanism of Ozonolysis by R. Criegee
- Advanced Organic Chemistry, 4th Ed. by Jerry March – John Wiley & Sons

Looks like they have a cyclic structure.