You heard correctly! From cosmic ray bombardments and particle accelerator experiments comes “muonium,” an other-worldly form of atom that, to a degree, resembles hydrogen. This atom is fascinating research scientists, along with another oddball, positronium.
An Exotic Element… Sort Of
There are some 90 naturally-occurring elements on Earth. There are more than a dozen other artificial elements, as well.
Muonium is artificial, but not in the usual sense of the term.
All of the more than one-hundred elements found in the periodic table consist of atoms made up of electrons traveling in orbitals about respective nuclei containing one or more protons and neutrons.
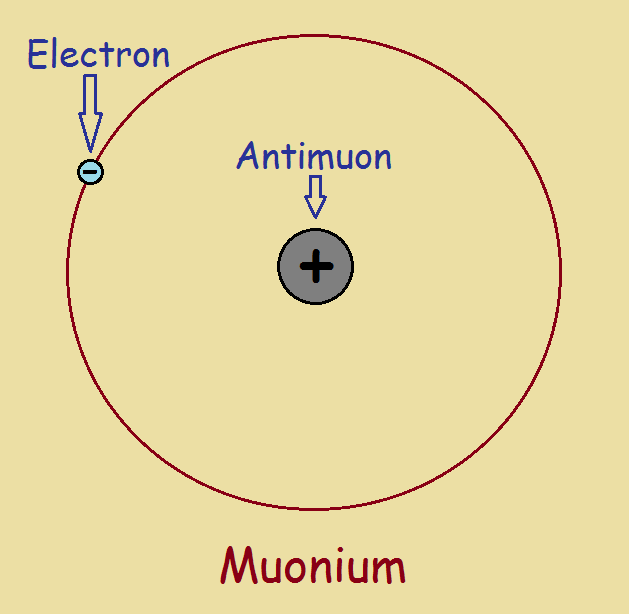
Though muonium does have an electron orbiting a nucleus, it possesses no proton or neutron. Instead, it’s nucleus consists of an antimuon, symbolized μ+.
To avoid confusion, we use the term “exotic atom.” This kind of atom includes the term “onium” in its name. Since the antimuon has a life of only 2.2 microseconds, one must act quickly to study this elusive “element.” Amazingly, science has uncovered enough information to allow publication of a book entitled “Muon and Muonium Chemistry.”
Note: The muonium we’re discussing here is sometimes mixed up with another substance called ‘true muonium.’ The two are different, and should not be confused.
Positronium and Muonium
Before researchers discovered the “atom” of our discussion, another artificial atom was the subject of intense study – positronium. Predicted in 1934 by a Croatian scientist, positronium was first discovered in 1951. Positronium consists of an antimatter positive electron (or positron) nucleus orbited by a regular matter negative electron.
In a very tiny fraction of a second, the positron and electron annihilate one another, yielding high-energy, gamma-ray photons. Amazingly, before its self-destruction, positronium can combine with ordinary atoms to form a hydride or other simple compounds.
Muonium was discovered later, in 1960. Assigned the chemical symbol Mu, we can compare this atom to the simplest form of hydrogen, with its one proton nucleus and a lone electron. Muonium is considerably lighter than hydrogen, the lightest of the elements. It weighs a mere 200 times the mass of an electron. The muon contains only 1/9 the rest mass of a proton.
Imagine (if its half-life allowed) the muonium equivalent of a hydrogen balloon that could lift much greater loads than an equivalent amount of hydrogen.
Where Do Antimuons Come From?
Where do antimuons come from? Cosmic rays, which include naked atoms (especially of the super-abundant hydrogen gas) – collide at terrific energies, leading through a series of preliminary decays to the formation of a muon – antimuon pair.A more productive method is to collide a positron (an antimatter positively charged version of the electron) with an electron.
Studying Hydrated Ions
Researchers have prepared and studied hydrated ions, such as the hexahydrate Mu(H2O)6+ plus one ion and compounds such as the chloride. Certain quantum mechanical properties involving the spin of the antimuon, which are beyond the scope of this article, have made these chemical studies possible.
“Another” Helium: Ongoing Research
Interestingly, a second “element,” muonium helium is now undergoing investigation, and is the object of careful research.
Note: You might also enjoy Do Lone Atoms or Molecules Assume States of Matter?
References:
- Walker, David . Muon and Muonium Chemistry. (1983). Cambridge University Press
- Fleming, et. al.. Kinetic Isotope Effects for the Reactions of Muonic Helium and Muonium with H2. (2011). Science
- Jungmann, K. Muonium. Cornell University Library
- Phillips, S. True Muonium. (2010). University of New Hampshire
- Fael, M. On the positronium contribution to the electron g-2. (2014). Cornell University Library
- Kirch, K. Testing antimatter gravity with muonium. (2014). International Journal of Modern Physics: Conference Series
- Core: Muonium Chemistry [Radiochimica Acta 26, 1-14 (1979)] by Paul W. Percival