LCAO MO
The nature of the chemical bond has been of interest for hundreds of years. Theories have been developed to explain how atoms combine to form molecules. The most successful theory to date is the (L)inear (C)ombination of (A)tomic (O)rbitals – (M)olecular (O)rbitals or LCAO MO bonding theory. You’ve got it: yet another acronym.
Atoms have nuclei. These contain protons and neutrons. Electrons travel around these nuclei. Orbitals are mathematical functions that describe their trajectories. As atoms form molecules, atomic orbitals combine to form molecular orbitals.
Since orbital functions are described by the Schrödinger wave equation and that equation is linear, molecular orbitals can be described by the linear additive combining of atomic orbitals.
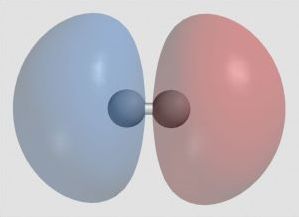
Example of Hydrogen
Ordinary hydrogen has one proton, no neutrons, and one orbital electron. Hydrogen forms diatomic molecules, H2. As hydrogen atoms approach each other, atomic orbitals combine to become molecular orbitals. They must be equal in number. Two atomic orbitals (1s) become one molecular orbital of lower energy (1σ, a bonding orbital) and one molecular orbital of higher energy (1σ*, an antibonding orbital). The bonding orbital is stronger than the simple atomic orbital, so H₂ is more stable than two atoms H. The antibonding orbital with two electrons in it would be less stable.
Filling the Molecular Orbitals
The Pauli Exclusion Principle states two electrons can fill one orbital if their spins are not the same. Since two hydrogen atoms possess only two electrons, the lower energy orbital is filled. The higher energy antibonding orbital is not filled. The result one molecule contains less energy than two atoms. The bond forms due to stabilization.
The Really Interesting Examples He2 and He2+
Helium has two electrons in each atom. To form a molecule, the four electrons must be placed in bonding and anti-bonding orbitals. If the energy from those two orbitals adds up to less than that of the two orbitals of atomic helium, a molecule forms. If not, it does not form. As it turns out, the molecule does not form. What is so interesting about that?
The He2+ ion does form! This is because there is less energy in two electrons in the bonding orbital and one in the anti-bonding orbital than in having three electrons in the atomic orbitals of two helium atoms.
Note: You might also enjoy Three Hydrogen Isotopes: Protium, Deuterium, Tritium
References: