- HO-CH₂-COOH → α-acetolactone + H₂O
- HO-CH₂-CH₂-COOH → β-propiolactone + H₂O
- HO-CH₂-CH₂-CH₂-COOH → γ-butyrolactone + H₂O
- HO-CH₂-CH₂-CH₂-CH₂-COOH → δ-valerolactone + H₂O
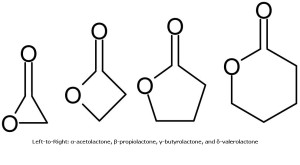
α-acetolactone
Known more simply as acetolactone, this molecule is unstable and hence transient. The synthetic reaction for acetolactone written above, would produce close to 100 percent of the desired product, if it were stable.
β-propiolactone
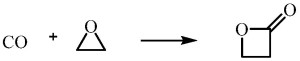
Sometimes called simply propiolactone, this molecule is stable, but is being phased out for many applications, since it is considered a potentially serious health risk and other materials can often substitute for it. One industrial synthetic method combines carbon monoxide and ethylene oxide.
γ-butyrolactone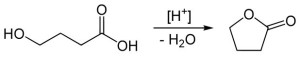
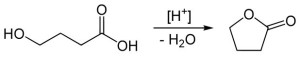
A much more useful compound is γ-butyrolactone. It is used as a reagent to produce other compounds, and is particularly useful as a solvent. In the body, this inactive lactone metabolizes into the biologically active γ-hydroxybutyric acid.
δ-valerolactone
The δ-valerolactone ring is useful in certain living, cationic polyester-formation reactions.
More Complex Lactones
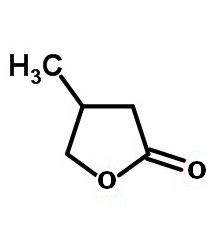
Engineering more complicated lactone structures requires involves reverse thinking – imagining the product, then going backwards to the starting materials. Pendant groups may be added in this way. For instance, the number 3 carbon could have one of its hydrogen atoms replaced by a methyl group (-CH₃). The result would be 3-methylbutyrolactone.
Note: You might also enjoy Organic Chemistry: What is a Lactam?
References:
← Back to Classic Science
← Home