Fortunately, when in close contact, atoms can react in a number of ways.
Often they link to each other in various combinations through bonding, forming molecules called compounds.
Such interaction requires explanation, and so provides employment to humans educated in this field: The field called chemistry.
Chemical Bonds: Ionic and Covalent
There are a variety of ways atoms bond to one another. Some bonds are weaker, and some are stronger. Two of the strongest forms of chemical bond are the ionic and the covalent bonds. Chemical bonds form between two atoms, each with its own electron environment.
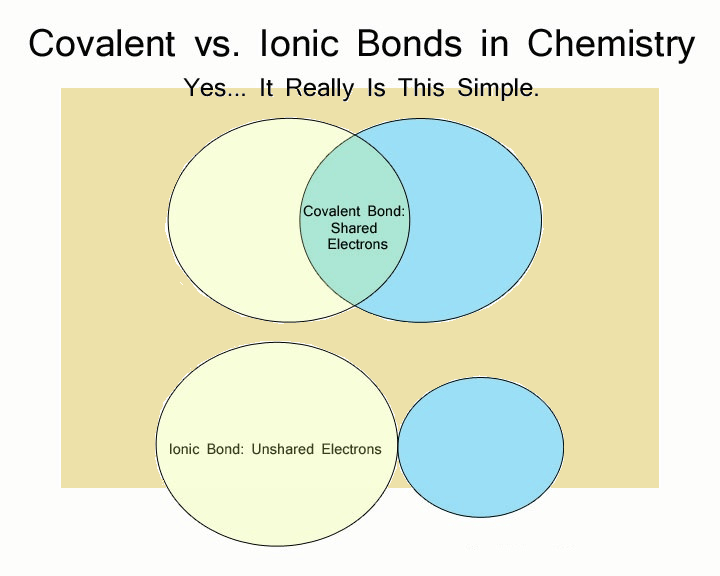
If each of the two atoms shares an electron with the other atom nearly equally, the bond is called covalent.
If one atom exerts considerable force over the other atom’s electron, while the other atom strives to give its electron over, the bond is largely ionic.
Which form of bond—covalent or ionic—is the stronger? The easy way to determine that is to measure the energy it takes to break the bond. That quantity is called bond dissociation energy. The greater the energy it takes to break the bond, the stronger that bond must be. It turns out that most ionic bonds are considerably more difficult to break than covalent bonds.
Ionic Bonds: Electronegativity
In the 1930s, Dr. Linus Pauling expounded on the quality he called electronegativity. Some atoms, if they can be humanized, desire to increase their electron density. Others desire the exact opposite. He developed a list of numbers that quantified that affinity. The presence of appreciable electronegativity favors ionic bond formation.
The simplest way to determine which of the ionic bonds are strongest is to examine the electronegativities of the anion (the negative portion of a compound) and its cation (the positive portion of the compound).
Alkali Metals and Halogens
The alkali metals are the least electronegative elements found in the periodic table, whereas the halogens are the most electronegative elements. We list three combinations of these elements, lithium iodide, potassium chloride and rubidium fluoride.
Lithium iodide————-352 kilojoules per mole
Potassium chloride——–427 kilojoules per mole
Rubidium fluoride———494 kilojoules per mole
Stronger than even these should be the ionic bond in the compound francium fluoride. It is the most electropositive of the elements. Hence, it follows that francium is the least electronegative element, as well.
Chemical Bonds and Electronegativity
In chemistry, we study the interactions between atoms. Elements stick together via chemical bonds; covalent and ionic, including Linus Pauling’s electronegative elements. Which bonds are the strongest? It depends on their properties.
Note: You might also enjoy What are Hybrid Atomic Orbitals?
References:
- University of Buenos Aires. Properties of Atoms, Radicals, and Bonds
- Linus Pauling. The Nature of the Chemical Bond. Electronegativity: Narrative. Oregon State University
← Back to Classic Science
← Home