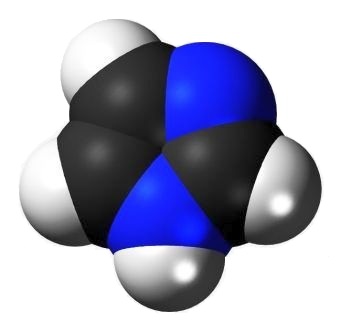
 Imidazole is an aromatic 5-member ring organic compound containing two skeletal atoms other than carbon. Both of those are nitrogen.
Imidazole is an aromatic 5-member ring organic compound containing two skeletal atoms other than carbon. Both of those are nitrogen.
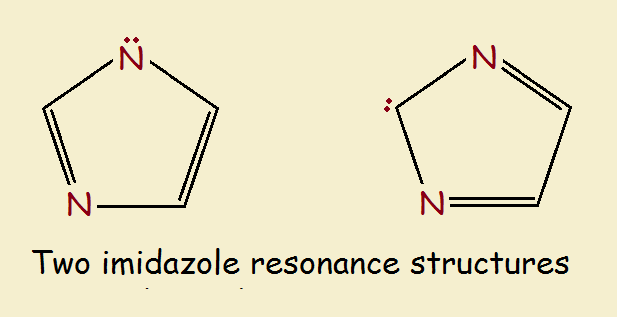
One of the molecule’s resonance structures, if it actually existed, would contain a secondary amine group (-NH-), an imine group (=N-), and an alkene group (-C=C-). The other resonance structure would contain two imine groups and a methylene group (-CH₂-).
Those structures are drawn below. However, imidazole doesn’t act like either of them. This is typical for aromatic compounds. We will briefly discuss the ring’s synthesis and chemistry.
 Synthesis
Synthesis
Imidazole is formed by reacting glyoxal with formaldehyde in the presence of ammonium acetate in acetic acid. The driving energy is microwave radiation. More generally, this reaction is used to produce substituted imidazoles. The basic reaction, however, is written,
OHC-CHO + HCHO + (NH₄OOC-CH₃ in CH₃COOH) + energy → C₃N₂H₄
In plain English this reads,
glyoxal + formaldehyde + (ammonium acetate in acetic acid) + energy → imidazole
As may be imagined, the two oxygen atoms of the glyoxal are replaced by nitrogen atoms from the ammonium acetate. This makes up most of the ring, which is closed by the formaldehyde structure, which also loses its oxygen atom.
Aromaticity
At first glance, it would appear imidazole is not aromatic, but the nitrogen atoms each have a pair of free electrons. By so-called electron or arrow pushing methods, it can be shown that one of the two pairs of free electrons is added to the resonance package and the overall molecule is indeed aromatic. Watch the Khan Academy video, cited in the references, for further evidence of that fact.
Chemistry / Importance
I first became acquainted with nitrogen heterocycles such as imidazole in connection with fuel-based detergents. These are designed to keep automobile engine parts clean and free running.
However, the most important applications involving imidazole are in biochemistry. This is due in part because of its water solubility. The nitrogen atoms interact with other biochemicals that seek to form hydrogen bonds.
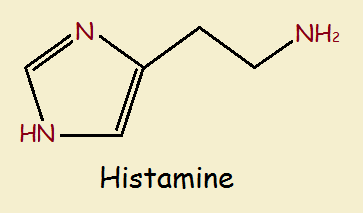
Consider the histamines, which feature the 5-member ring. Basic histamine is nothing more than a molecule of ethylamine (CH₃CH₂NH₂) attaching to a molecule of imidazole by replacing one of the hydrogen atoms attached to a carbon.
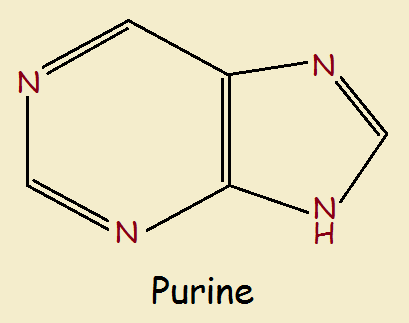
The most common biological molecules that contain the imidazole ring are the purines. Its 5-member ring is fused to a 6-member pyrimadine ring. It introduces two more nitrogen heteroatoms. See below.
Note: You might also enjoy Forming Nitrogen Heterocycles from Aliphatic Amino Acids
References:
- Der Pharma Chemica, 2012 4 (1): 116-140: Review: A. Chawla, et. al.
- Khan Academy: Aromatic 5-Member Heterocyclic Rings
← Back to Classic Chemistry
← Home
