Abstract: The water molecule, H2O, can attach a hydrogen ion (H+) perhaps from a dissolved acid, to become a hydronium ion, H3O+, sometimes called a hydroxonium ion. This ion, if surrounded by water molecules, can form additional hydrogen bonds with them as well. The question is what is the molecular structure of the resulting hydronium ion hydrate? The mind conceives two serious possibilities. But what do studies reveal?
Background
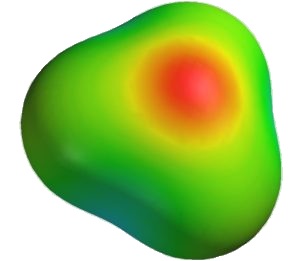
It is a general principle of nature that a system is more stable if charges are spread out as much as possible. In the field of chemistry, the best known example of this involves water. Although the sum total of the electric charge of a neutral water molecule is zero, as the image included here reveals, if an “axis” is drawn along the length of the molecule, on the one side of the axis is a slight positive charge, while on the other side there is a slight negative charge. This is because water is bent 104.45°.
Attraction
The full positive charge of a hydronium ion is drawn to the partial negative charge of a water molecule. The end result is the formation of a new kind of chemical bond, not as strong as a full ionic chemical bond. It is called a hydrogen bond. In this instance, since the hydrogen bond forms between an hydronium ion and a water molecule, we call it hydronium ion hydrate (image right and below).
Keep in mind that a hydronium ion possesses a full ionic charge and may draw more than one water molecule to itself. In fact, it is a very real possibility that as many as 20 molecules of water associate with one ion. Others suggest a number of about 5 or 6. To spread a lone charge out over so many atoms would truly comprise a stabilizing dispersion of charge!
The Eigen Concept of Hydronium Ion Hydrate
One intriguing suggestion is the Eigen concept. In it, the hydronium ion is surrounded by three water molecules, H9O4+. This mosaic is not flat, however, but shaped like the upper half of a skull, which, if our image is viewed from above, shows the outermost hydrogen atoms lying furthest away from us. And if much of the dispersed electrical charge is carried by the outermost atoms, there are six hydrogen atoms at the periphery which would be more than “willing” to accomplish the task.
In Conclusion
While the smaller structures of 5 or 6 water molecules might seem more reasonable to some, the idea of increased stability appeals to a greater numbers for maximum dispersal. In fact, perhaps the entire mass of fluid present could be viewed rather like a sea of molecules, making and breaking connections much like some kind of Tarzan swinging from one grapevine to another, forming various-sized ionic structures, dispersing as much electrical charge as it can.
Note: You might also enjoy “What is a Hydronium Ion?”
References:
- Water Structure and Science – by Martin Chaplin: Hydrogen Ions
- Springer Nature Chemistry: Direct Observation of a Hydronium Ion Coordination by a Proton