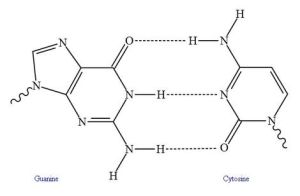
Hydrogen bonding ranges from simple interactions to protein folding and the double helix of DNA.
Atoms bond to each other in a variety of ways. Among these are ionic bonds. Ionic bonds feature electron transfer between atoms. Then there are covalent bonds. These share electrons between atoms. There are also and metallic bonds. These consist of a matrix of atoms interlaced with mobile valence electrons.
In addition to these major forms of bonding, there are weaker yet highly important bond types. Foremost of these are hydrogen bonds.
Description
A hydrogen bond is a tenuous electrostatic attraction between an already bonded atom of hydrogen and a second (electronegative) atom. These two atoms can be located in the same molecule or different molecules.
Bonding between different molecules, it is intermolecular. Bonding within the same molecule, it is intramolecular. The length of a bond suggests its strength. A strong bond is short. Hydrogen bonds are shorter than van der Waals bonds. But they are longer than covalent and ionic bonds.
Intermolecular Hydrogen Bonding
The foremost example of H-bonding is water. A hydrogen atom of one water molecule bonds to the oxygen atom of another water molecule. The second hydrogen atom can bond with the oxygen of a third molecule. And so on.
Interestingly, under the special conditions of freezing and pressurization, all hydrogen to oxygen bonds in water can be made to approach equality. This is called symmetric hydrogen bonding. In this, each bond possesses a measure of covalent character.
Intramolecular Hydrogen Bonding
An example of intramolecular bonding is 2-cyanoethanol, HO-CH2CH2-CN. The hydroxyl hydrogen (the H of the HO- group) is electrostatically drawn to the nitrogen of the cyano group (the N of the -CN group).
The result is a loosely flexible six member ring with the skeleton H-O-C-C-C-N. The other hydrogen atoms are attached to the two central carbon atoms. Intramolecular bonding can position atoms to favor one kind of reaction over another.
General Effects of Hydrogen Bonding
Since intermolecular hydrogen bonds bring molecules closer together, liquids increase in density. It requires energy to disrupt these bonds, so the liquids have a higher boiling point.
Hydrogen bonds, though flexible, distort molecules into specific positions. Hydrogen bonded compounds experience greater water solubility.
Polymer and Macromolecule Effects of Hydrogen Bonding
In biologically active macromolecules, hydrogen bonding contributes to more complex levels of structure. These include protein folding. And parallel hydrogen bonding (H-bonding between two linear structures) increases strength in fibers such as cellulose.
Note: You might also enjoy The Molecular Structure of Hydronium Ion Hydrate
References:
- Definition of the Hydrogen Bond
- Intramolecular Hydrogen Bonds in 2-Cyanoethanol and in Some Nitroalcohols
← Back to Classic Science
← Home