 When doing the laundry, we ask, what temperature should the water be, how much detergent should I use, will I need fabric softener, will I need bleach?
When doing the laundry, we ask, what temperature should the water be, how much detergent should I use, will I need fabric softener, will I need bleach?
If I use bleach, should I use chlorine bleach or should I use oxygen bleach?
Kinds of Bleach
There are two kinds of bleach, based on needed strength and fabric sensitivity. Chlorine bleach, historically the older and stronger variety, is based on sodium or calcium hypochlorite, NaOCl or CaOCl. One name brand of laundry bleach is Clorox®. It contains 5.25% NaOCl.
How does chlorine bleach remove color? In order to understand that, we need first to ask, what is the chemistry behind the colors used in fabrics?
Color in Fabrics
When we think of colors applied to fabrics, the chemist usually thinks of chromophores. This topic is discussed in the QuirkyScience article What’s a Chromophore?
Chromophores are generally associated with carbon atom chains with a collection of alternating or conjugated double bonds.
Chlorine Bleach and Double Bonds
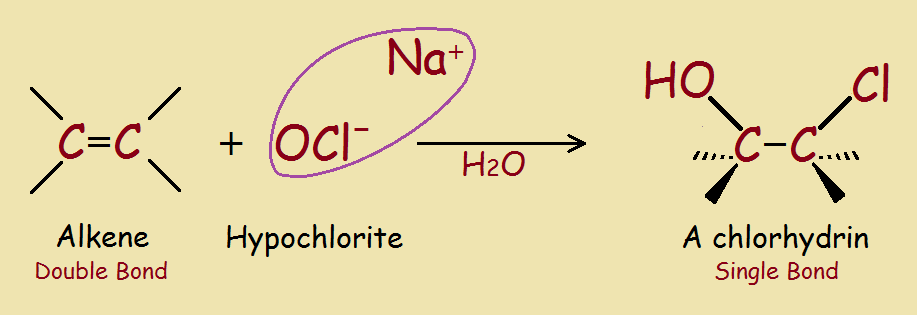
The chemistry needed to remove color is not complicated. Remove enough double bonds and you remove color. What is a simple way to remove double bonds? Convert them into single bonds. Adding atoms to both sides of a double bond accomplishes the task (see the image¹).
Next: What is Oxygen Bleach?
What are the active components found in oxygen bleach? Compounds having a close connection to hydrogen peroxide; for example, sodium percarbonate, 2Na2CO3·3H2O2 and sodium perborate, Na2(B2O4(OH)4). We can understand their behavior if we understand the behavior of hydrogen peroxide, itself.
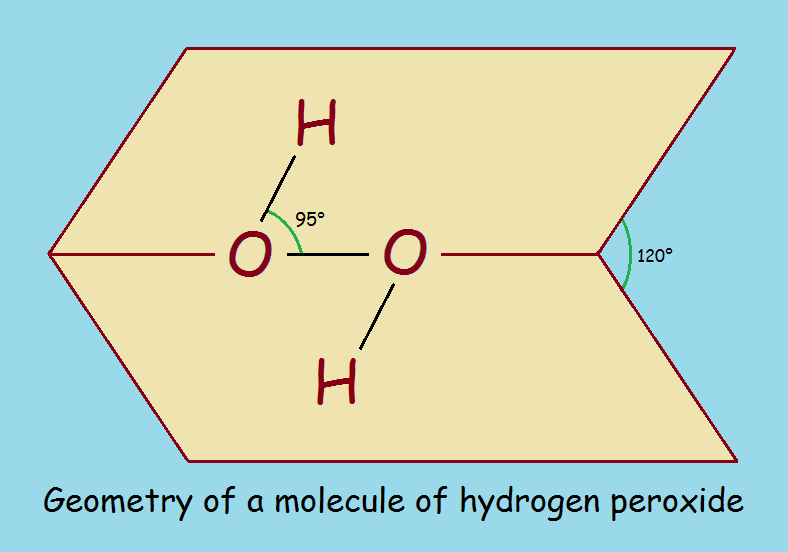
Hydrogen peroxide decomposes in two ways…
H2O2 → 2 HO•
H2O2 → H2O + [O]
In the first reaction, hydrogen peroxide breaks into two hydroxyl free radicals.
In the second reaction, we have the production of atomic oxygen. Interestingly, this second decomposition reaction plays but a very minor role in the bleaching process.
What Happens?
Unfortunately, the exact bleaching mechanism, both for hair and for clothing, is not known with certainty. It appears the hydroxyl free radicals react with additional peroxide to produce as the probable culprit, the hydroperoxyl radical, HO2•.
We write,
HO• + H2O2 → HO2• + H2O
These radicals react with organic colorants in textiles. How?
Chemistry of Color in Fabrics
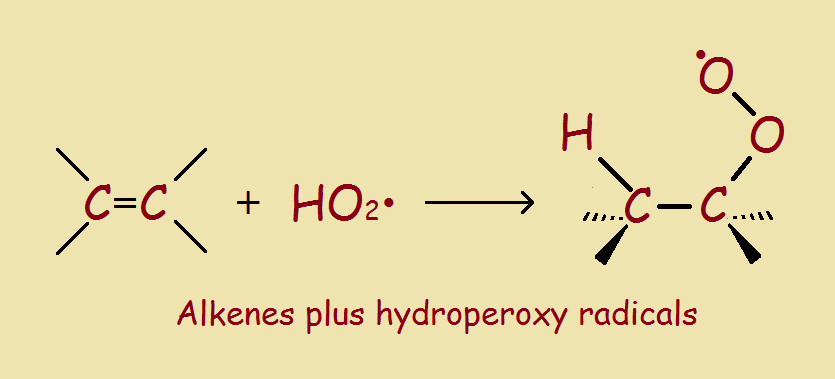
When we think of colors applied to fabrics, the chemist usually thinks of chromophores. This topic is discussed in the QuirkyScience article Chemistry: What’s a Chromophore. https://www.quirkyscience.com/chromophore/.
Chromophores are generally associated with carbon atom chains with a collection of alternating or conjugated double bonds.
Logically, the removal of color must involve the interaction of hydroperoxyl radicals and conjugated double bonds. See our final image to understand the reaction sequence for oxygen bleach. The end result is the same: double bonds, chromophores, and stains, bite the dust.
What must surely be obvious is that the bleaching action of oxygen bleach is less than that of chlorine bleach. Otherwise, chlorine bleach would be the choice for delicate colors and fabrics.
1 The image: The reader may notice the sodium ion (Na+at left) is missing at right. Minor pH factors explain that, but including them would only muddle the concepts.
Note: You might also enjoy Organic Chemistry: What is a Lactam?
References:
- Royal Society of Chemistry: Did You Know? About Hydrogen Peroxide
- The Mechanism of Hydrogen Peroxide Bleaching by Spiro and Griffith
- Journal of the American Chemical Society: Addition of Peroxyl Radicals to Alkenes and the Reaction of Oxygen with Alkyl Radicals
← Back to Classic Science
← Home

Bleach is a very useful item, though I try not to use too much, so it doesn’t affect the water in the drain.
The cool thing about chlorine bleach is it breaks down into salt and oxygen.