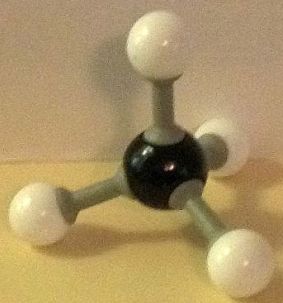
Methane is the smallest molecule consisting of just carbon and hydrogen, CH₄. The second smallest hydrocarbon is ethane, C₂H₆. It is sometimes written H₃C–CH₃. The “dash” between the two carbon atoms represents a single bond. It is also a sigma bond. What is a sigma bond? And what are ethane sigma bond rotation conformers?
What is a Sigma Bond?
The carbon to carbon single bond is a covalent bond. That is, bonding electrons lying between the two carbon atoms are shared equally. A sigma bond is the strongest variety of covalent bond. The two atoms overlap head-on. One could view such a bond as being of cylindrical symmetry or like a straight line between the two atoms. Hence, the dash notation. Since the overlap is essentially a force, the atoms can, unless prevented by other factors, rotate with respect to each other about that bond.
Each of the two carbon atoms has three hydrogen atoms bonded to it, equally spread about, so the –CH₃ groups resemble propellers. These methyl group “propellers” can spin about the C–C sigma bond. While they are not prevented from so rotating, all the possible conformations the two groups can attain are not equal. Some force the molecule to take on a little greater energy. Others allow its release.
Rotation About the C–C Sigma Bond
Chemists us a variety of mechanical models to visualize physical properties of atoms and molecules. For ethane’s rotation about the C-C sigma bond, the following YouTube video employs both the space-filling models and the ball and stick model
Ethane Sigma Bond Rotation Conformers
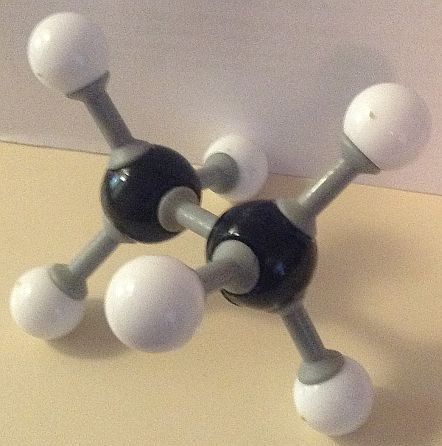
Here are our ball and stick model images of the lowest and the highest energy conformers of ethane. Each hydrogen atom is surrounded by a small cloud of negative electrons.
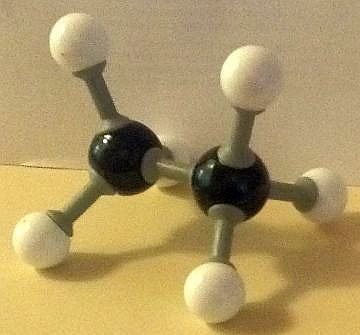
The potential energy of the conformer with all six hydrogen atoms in closest proximity (the eclipsed structure) to each other is the greatest, due to repelling forces being at a maximum. This makes it the least stable conformer. The conformer with the hydrogen atoms furthest apart (the staggered conformation) possesses the least potential energy and so is the most stable.
Note: You might also enjoy Hückel’s Smallest: The Aromatic Cyclopropenyl Cation
References:
← Back to Classic Science
← Home

I love the way you make chemistry seem a lot simpler than it used to appear back in my school days!