But what is it that makes epoxy glue so strong? Its name gives it away. Epoxy glues use epoxy compounds. Epoxides. What is an epoxide?
It’s Simple? Yes
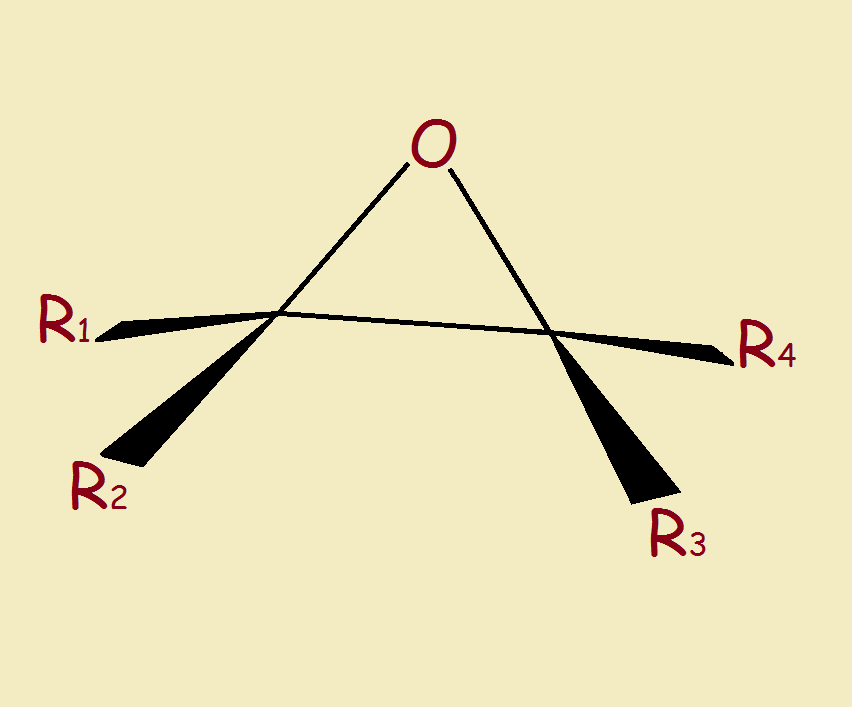
An epoxide is a molecule with a tiny 3-member ring in its structure. One atom is oxygen. Two are carbon. The epoxy link is illustrated in Figure 1. R₁, R₂, R₃, and R₄ are pendant groups. How is such a 3-member ring created? Often by the oxidation of an alkene. The reader may appreciate an example.1
Propene
Let’s try the oxidation of propene, CH3CH=CH2.2 What would be a suitable oxidizing agent for the reaction? It is not practical to use elemental oxygen. The violent conditions that would be needed in order to divide an O2 molecule into two O atoms would destroy the alkene.
Since we seek to add a lone atom of oxygen to the double bond in the mildest way possible, the logical choice is some kind of unstable peroxide. Why? A peroxide is unstable and wants to eliminate an oxygen atom anyway. For each molecule of H2O2 used, one O atom is released, giving water, H2O.
The reaction is written,
H2O2 → H2O + [O]
Unfortunately, most organic compounds do not dissolve in water or H₂O₂. So, an organic peroxide is needed. Occasionally, rather than a peroxycarboxylic acid may be used instead.
RCO2OH → RCOOH + [O]
Let’s Boogie
The reaction we will examine uses peroxyacetic acid and propene gas. See Figure 2. Reaction products are acetic acid and the epoxide of propene—propylene oxide. While there are more practical commercial methods, this simple reaction is instructive. But, once prepared, of what value are epoxides (also called oxiranes)?
Commercial Epoxide Use
Epoxides afford what practical uses? The ring is fairly unstable. It easily opens in a host of reactions. Although the most common use for epoxides is in epoxy resins, it is by no means their only use.
Important in Synthesis
Perhaps the most valuable epoxide is the simplest one, ethylene oxide, (CH₂)₂O. It is of no little importance in the laboratory to the synthetic organic chemist.
1 Symbols for the elements discussed in this article are, for carbon, C, for hydrogen, H, and for oxygen, O.
2 Propene is more commonly called propylene.
Note: You might also enjoy Lactones: What Are They? How Are They Made?
References:
- Organic Chemistry: Hydroxylation and Oxidation of Alkenes
- Polymer Science Learning Center: Epoxy Resins