 There is a lot of talk concerning the chemistry of carbon. And this is totally appropriate. The chemistry of carbon is the chemistry of life. But little is known about boron, which is right next door to carbon on the periodic table of the elements. Yet, boron is a most interesting element. For one thing, boron, like carbon, is capable of bonding to itself.
There is a lot of talk concerning the chemistry of carbon. And this is totally appropriate. The chemistry of carbon is the chemistry of life. But little is known about boron, which is right next door to carbon on the periodic table of the elements. Yet, boron is a most interesting element. For one thing, boron, like carbon, is capable of bonding to itself.
Boron Bonds to Itself
There are many compounds in which the element does just that, it bonds to itself. Consider a few of its combinations with hydrogen (see the image). As you advance to larger boron-hydrogen structures, however, it becomes clear the molecular bonding for boron differs considerably from the hydrocarbons.
Dodecaborane
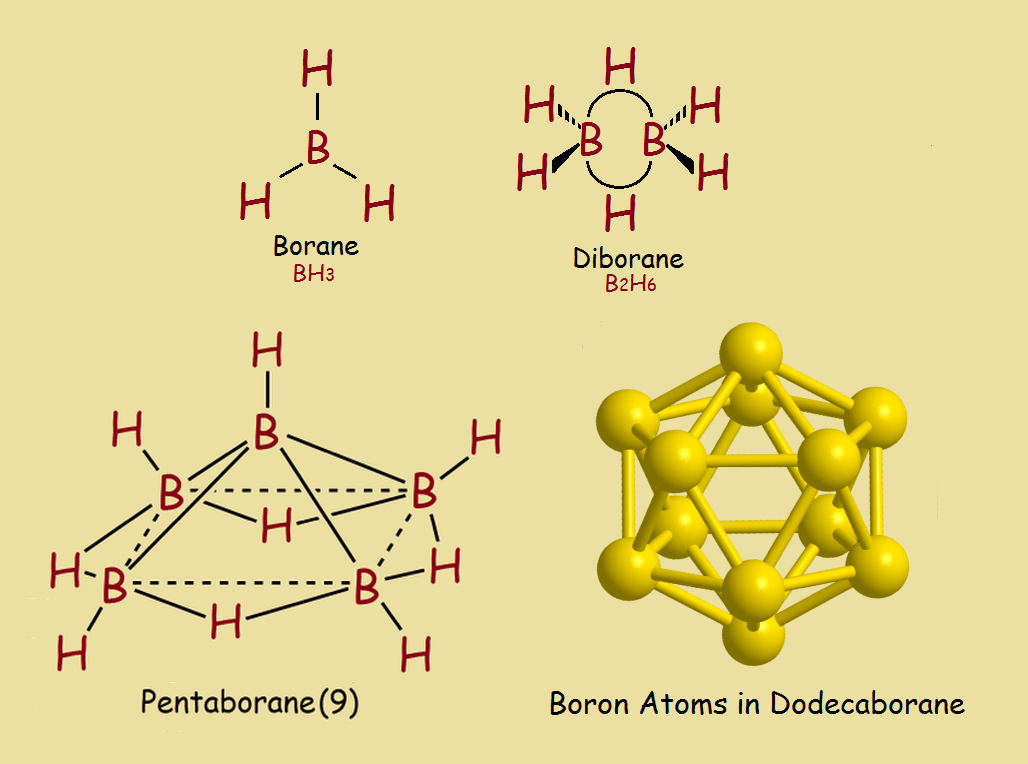
Dodecaborane somewhat resembles dodecahedrane (C20H20) in outward appearance, even though in terms of bonding, there is considerable difference. Also, the names mislead somewhat. In dodecahedrane, there are 20 carbon atoms, whereas in dodecaborane, there are only 12 atoms of boron.
The reason is the dodeca- part refers to the number 12. There are 12 boron atoms in dodecaborane. There are 12 surface pentagons in dodecahedrane.
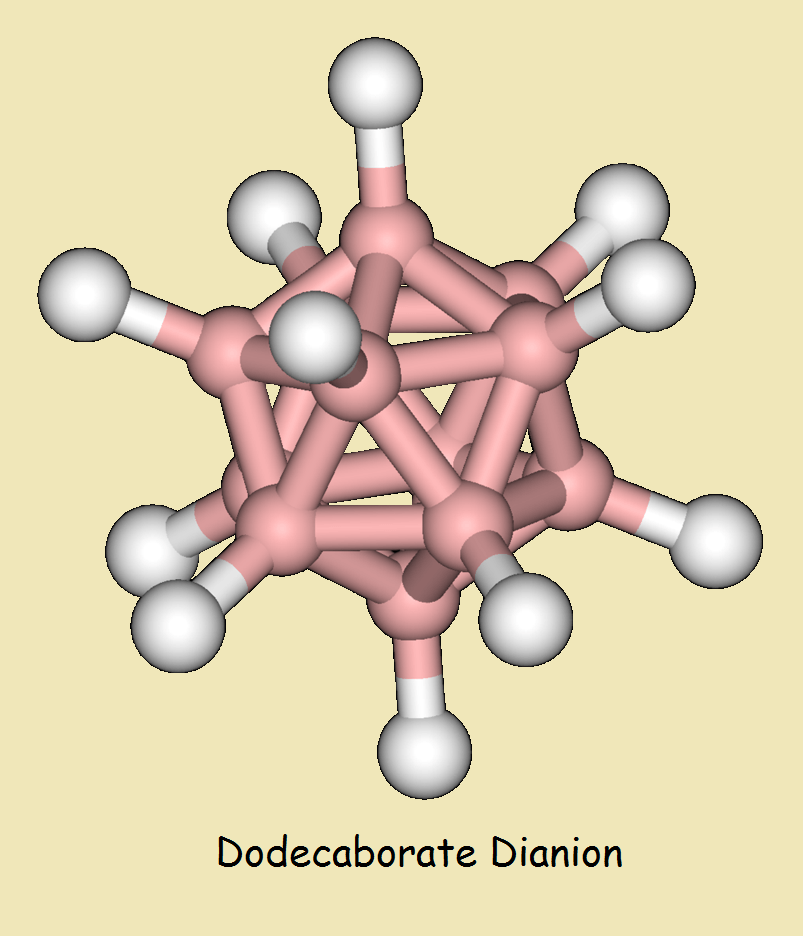
Dodecaborate Dianion
Interestingly, both borane and diborane are unstable molecules. One would suspect that, if the precursors of larger boranes were unstable, so would be their larger analogues – if they could be synthesized at all.
Despite that, theory suggested the existence of a stable icosahedral dianion of dodecaborane as early as 1955. Theory was proven by synthesis in 1960. The chemical formula of dodecaborate dianion is written,
[C12H12]-2
As might be anticipated, such a complex compound is possibly useful for equally complex applications. However, those applications are so complex and, perhaps it could be said, so strange, they won’t be discussed here. If the reader is interested in pursuing its applications, we suggested he or she “search Google”. Note: You might also enjoy Chemistry: What’s a Chromophore? ← Back to Quirky Science Nuggets
← Home
