
I was in my 30s when I first heard of Devils Dice. What are they?
I was raised in New Jersey. At the age of 33, I moved to Virginia. This short north-to-south emigration exposed me to certain speech, food, and culture changes. It took me a month to get over the accent. During that time, my brain adjusted and the accent sounded totally normal to me! I missed scrapple a lot. Since then, I’m happy to say scrapple successfully has invaded the south.
But there were other changes as well. In the south, there are buzzards. There are red bud trees. There are ABC stores! Even the ground itself offered something new. There is this kind of rock on the surface of the soil nicknamed Devils Dice. It starts out a beautiful brass color, but weathers over time. It turns a dark chocolate brown. What are Devils Dice? Why and how do they change color?

Devils Dice a Modification of Pyrite
Pyrite is an ore of iron, in fact the sulfide of iron. One atom of iron (or ferrum, Fe) combines with two atoms of sulfur (S), to form FeS₂. It can assume cubic, pyritohedra, or octahedral crystalline forms. Devils Dice earned its name because it is the cubic variety of pyrite. Interestingly, during crystallization, the competing of the crystal types can introduce facial lines, facial striation.
Cubic Form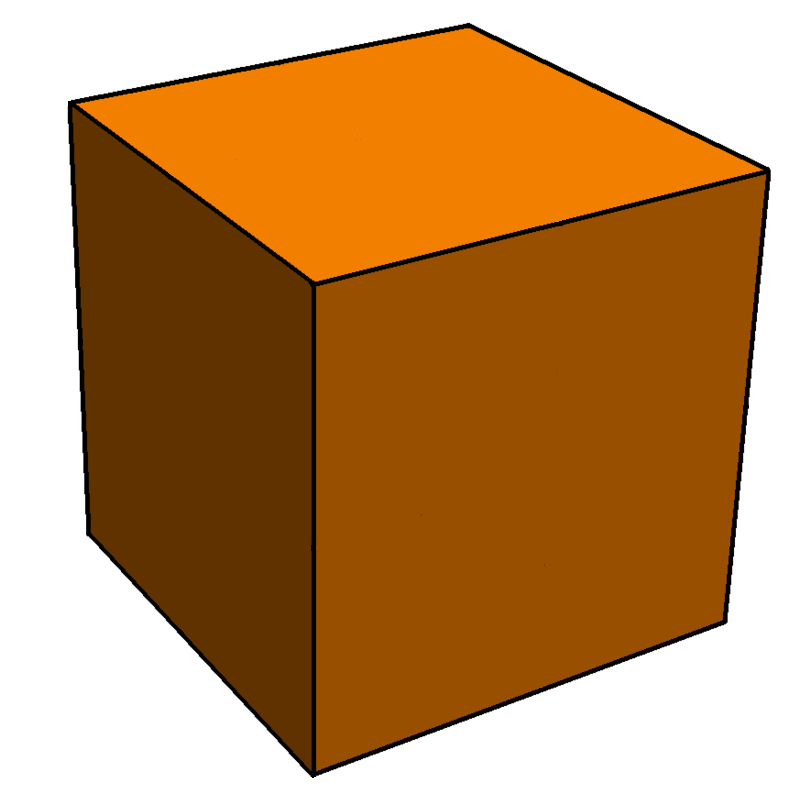
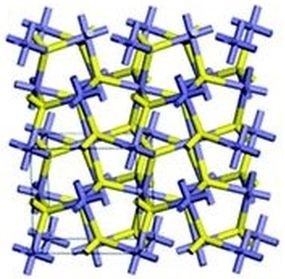
Molecules of iron pyrite connect in such a way that a cube (or a cuboid) easily results. In the schematic layout shown, notice the cube in the lower left quadrant. At the center of that cube are two sulfur atoms. The rest of the layout follows naturally. Iron atoms are seen at each corner and in the center of each face of the cube.
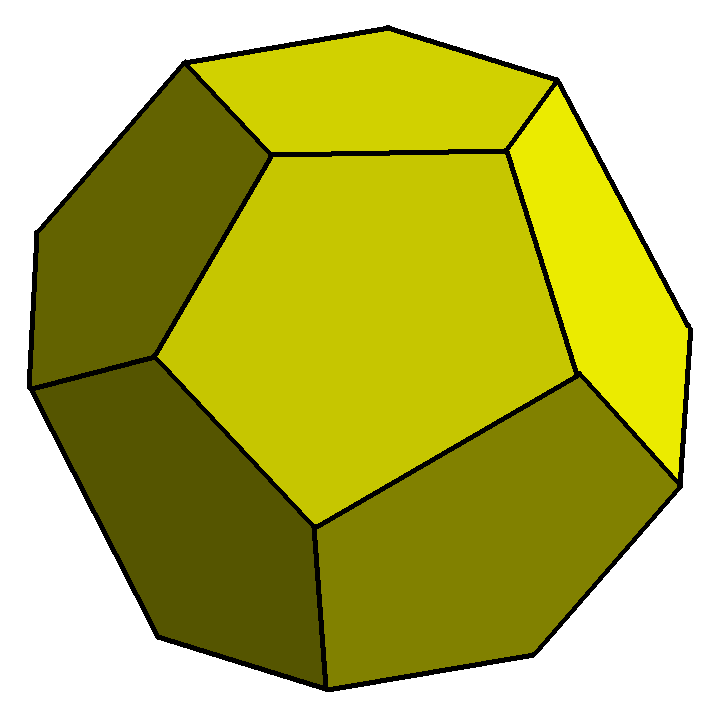
Pyritohedron Form
How is it that pyrite can form a number of crystal shapes that are as different as the cube and the pyritohedron? It is because they are not actually that different. A cube is divided into a pyritohedron by bisecting all its edges and faces in alternate directions.
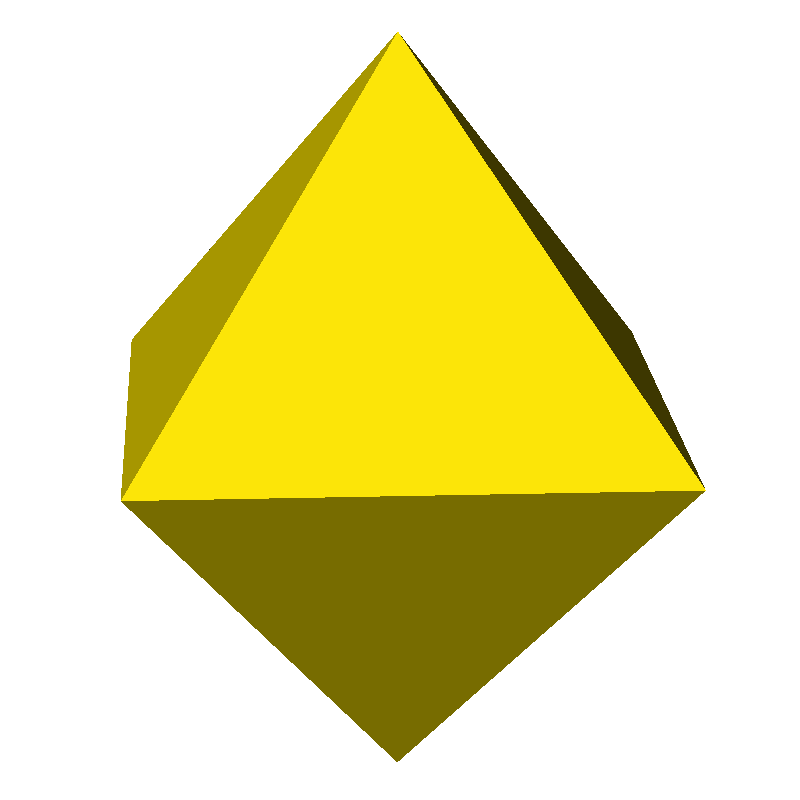
Octahedral Form
The above explanation of why a pyritohedron doesn’t represent a great departure from the cubic is quite valid. And the same can be said of the octahedron, as any iron atoms are at the corners and/or faces of the cubic crystal lattice. Yet the reason the three different forms (including the very rare octahedral) form has to do with concentration during the time of formation. Nucleation depends upon that concentration, and the manner of nucleation determines the crystal structure formed (see reference, below).
Transformation by Weathering
Many metals tarnish. We might be inclined to think the presence of air is all that is required to produce a tarnish. In fact, the presence of water, and not air alone, greatly hastens tarnishing. Cubes (and other shapes) of pyrite react with these to form a chocolate brown tarnish or coating called limonite. What is the chemistry of limonite?
The chemical formula of Limonite is FeO(OH)·nH₂O. In other words, the oxygen and hydrogen of the air and moisture have replaced the atoms of sulfur in the outer layer. The limonite remains bonded to the pyrite at their interface. It’s rather like a skin has formed over the pyrite.
An overall reaction equation could be written,
4 FeS₂ + 10 H₂O + 15 O₂ → 4 FeO(OH) + 8 H₂SO₄
But where are the n molecules of water that form part of the limonite structure? They can easily be accounted for by including an excess of water on the left side of the chemical reaction.

Practical Pyrite Uses
One might naturally think more in terms of the iron atom in the pyrite molecule, but there are twice as many sulfur atoms, and pyrite was once a predominant starting material for the production of sulfuric acid.
Curiously, the name pyrite suggests another real use. Pyrite, struck against some other hard material, such as metal, can be used to produce the sparking necessary to initiate a fire. This property should be of special interest to the survivalist.

Pyrite is also used, polished, as an ornamental stone.
Note: You might also enjoy The Devils Walking Stick – Aralia Spinosa
References:

Sounds like you could make use of this as jewelry that would have a practical use in a survival situation!
I was born in 1968. I remember these as a child. I found them when I was digging on Frederick Circle, near Barracks Rd., in Charlottesville, VA. I was just thinking about them, and when I looked it up I was so pleased to find your article. Definitely a memory for me! I love that we used the same name, Devil’s Dice.
This is SO funny. You see, I first found Devil’s Dice in Charlottesville!
What a coincidence! I was born in 1967 and found devils dice in Charlottesville when I was about 7 (1974) We lived near 5th St, Old Lynchburg Road I think. They just sat on top of the red clay after a rain. I’ve never seen them anywhere else.
The very place I found them!
Beautiful. I thought it was pyrite when I first saw the picture.
And, indeed it was. But a particularly interesting form. Apparently not as widespread as I might have thought!
I searched for “Devil’s Dice” on a whim today trying to figure out what sort of rock it was I carted around the country for years as a kid. I dug mine out of the hill my elementary school (Rose Hill Elem.) sat on, also in Charlottesville, VA, in 1967-68. My most treasured find was about one inch square and very heavy. Absolutely tickled to see there were other kids my age digging for the Devil’s Dice during the same time frame.
Once, in the property of the resident at South Haven Acres Lane, off Old Lynchburg Rd, in C’ville, I discovered a huge devil’s dice. Bigger than a showpiece at the University of Virginia. Something about 3-1/2 inches by roughly an inch-and-a-half square. I behaved less than sensibly. I was under the impression the site was an Indian burial site. I attempted to hit the object to see if it was gold. It was gold alright… It shattered into an endless number of pieces. If you only knew how bad I felt about that!
I found them when the woods and fields were prepared for the building of Charlottesville High School. It was just one gigantic field filled with them.
I have the world’s largest collection of Devil’s Dice, each of which I picked up in various spots around southern Albemarle over the past 40+ years.
Cool! Have you ever photographed them, perhaps putting an image up of them online?
I have many of these collected from South Carolina. Can the brown tarnish be removed?
I’m sure it is possible, but what rock hound would wish to disturb the lovely chocolate patina?
They are beautiful looking. I did not realise that they shattered when hit. It would be fantastic to see a field full of them, though I suppose a farmer might view it differently!
I too knew “devil’s dice” from digging in the playground at Rose Hill Elementary school in Charlottesville about 1967-68. I am just stunned to see so many responses about DD from Charlottesville! I wonder if there is some unique geology in Albemarle County that made them so prevalent?
I am inclined to agree with you that conditions must have been “just right” for that. Amazingly so.
I’ve found a few pieces of devil’s dice in my backyard. I was just thinking it would be fun to get the kids a metal detector to look around the yard and the woods behind the house. Where’s my backyard? Charlottesville.
Sounds like fun. Invite me, too! Just kidding. You know, some geologist should made a podcast or host a show on why Charlottesville seems to hold the world’s supply of DD.
This is great! My sons (5 & 7 years old) just found dozens of Devil’s Dice this past weekend. My wife and I went online to learn more about these treasures and happily found this site. It will be no surprise to anyone reading this: we live in Charlottesville!
Dang, I live in Charlottesville, never heard of or seen these but I’ll be looking now!
I used to have a bucket full of Devil’s Dice that I had dug up in Johnson Village in Charlottesville. There was new construction there (we’re talking 50 years ago now) and the place was covered in them. I found my best one on the lot where, a couple of years later, my mom’s house was built. Alas, my mom got rid of them sometime after I left for college. I wish I still had a couple of the larger ones.
A bucketful! Wow. I first spotted mine not too far away where the local Kingdom Hall was being built. A few miles away from Johnson Village. I could have beat myself half to death when I realized a handful of miles further away from that, I found one about 4″ longs and an inch-and-a-quarter square that I broke. It twas bigger than the one at the science library of UVA! Sigh. Thanks for your comment, Sarah.
Wow, my family and I just found a handful or so by the Briar Fork Store. We figured we’d leave the rest for everyone else. Wonderful day out!
AKA ‘fool’s gold’. Wikipedia’s article on DD/ iron pyrite is worth a look.
I have 2 little ones found in western Albemarle Co. Being a native Virginian, I brought them and a clear chip of crystal quartz from the same place along as touchstones. It’s not at all rare and historically was used to produce sulfuric acid.
My dad, now 86 said he found his behind MacIntire elementary School in Charlottesville in the mud slide after a rain.