There is an endless number of theoretical molecular possibilities in the world of organic chemistry. Some chemicals are simple. Others are complex. Some are of practical use to mankind. Some are mere toys of the intellect. Yes, adults must have their toys!
Cubism
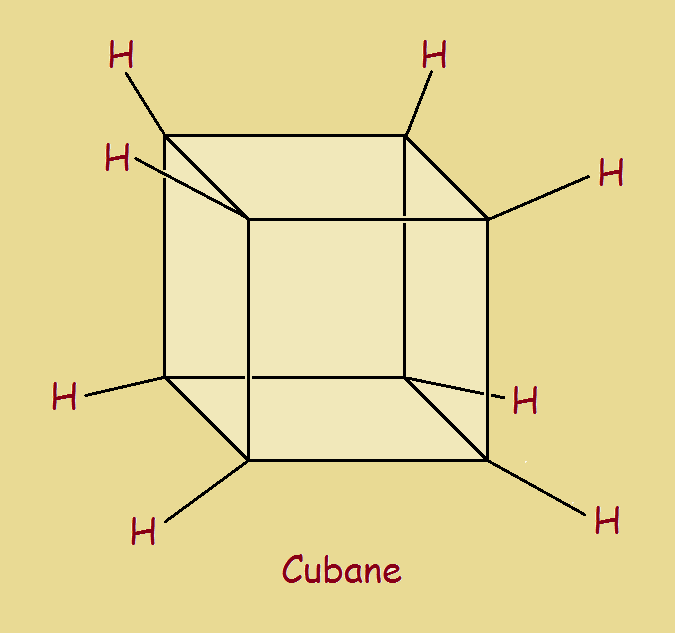
“We do the difficult immediately. The impossible takes a little time.” Chemists enjoy challenges. One challenge was to synthesize cubane (C8H8). It is a cut with a carbon atom at each corner. Attached to each of them is one hydrogen atom (see the image).
Now carbon “prefers” to form angles of 109.5 degrees. However, the angles of C8H8 are the same as those of any cube—90 degrees. The carbon atoms are strained nearly 20 degrees from their ideal configuration. It takes energy to force the deviation. Increase energy and you decrease stability.
Finally Synthesized
For that reason, it wasn’t until 1964 that cubane was finally synthesized. The chemistry may look tough. But what was tougher was to foresee what might work in the first place! Would you like to know how to prepare the cubic hydrocarbon? Let the Imperial College of London enlighten you. Once prepared, cubane’s properties were of paramount interest. Would C8H8 be useful for anything?
Explosive Possibilities?
Most hydrocarbons with only eight carbons would be liquids. Cubane is a solid. It is a waxy, rhombohedral crystalline solid. The hydrocarbon is surprisingly stable, despite bond strain. This may be because of its high degree of symmetry.
Cubane has a remarkable density of 1.29 grams/cm³. Although stable, it is very reactive. For this reason, there should be C8H8 derivatives well suited for explosives. One theoretical possibility is octa-nitrocubane. But that is one toy this chemist has no desire to synthesize!
Cubane Medications?
It is believed C8H8 derivatives may be useful in treating cancerous tumors, HIV, and AIDS.
Cubane Polymer Optical Displays?
Optically transparent, yet rigid cubane polymers may be well suited for liquid crystal design, hence optical displays.
Note: You might also enjoy Tetra-Tert-Butyl Methane – the Acyclic Alkane That Seemingly Should Exist
References: