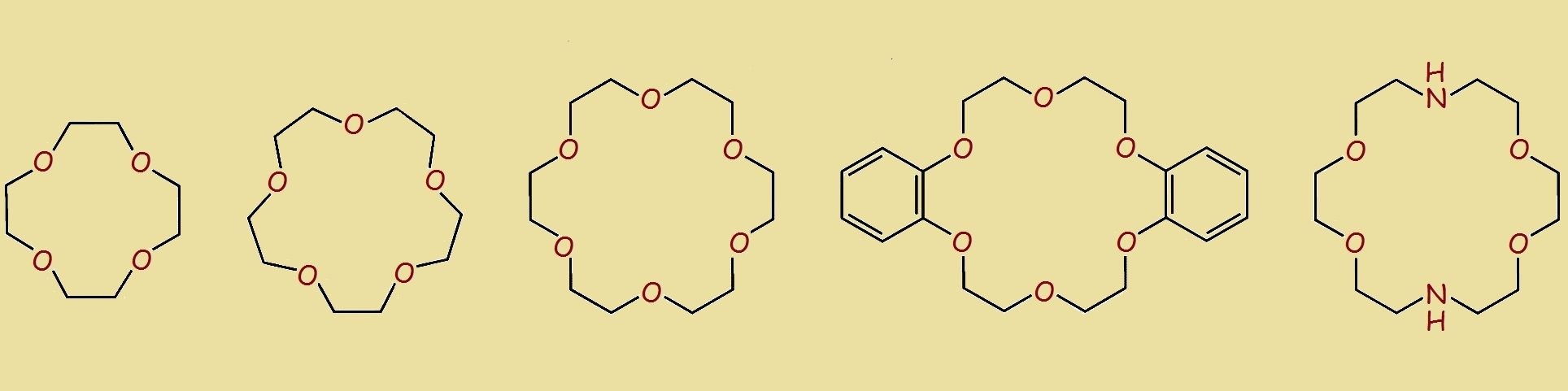
Crown ethers are designer molecules – ring structures intended to serve specialized purposes.
Each ring contains a number of ether linkages (-C-O-C-) that, based upon their structural formulas as they are drawn on paper, give the appearance of a royal crown.
The “crowns” have different “sizes.”
The most common crown ethers sport ethyleneoxy groups (-CH2-CH2-O-) joined end-to-end (making these structures oligomers), which are closed like a necklace.
Ring properties and rigidity may be modified by means of appendages on the ring or other hetero atoms along the ring.
Simple crown ether rings such as those shown in the figure lack rigidity because every bond is single. There are no multiple bonds reducing flexibility.
Crown Ether Nomenclature
For the most common crown ethers, there is a simplified nomenclature that first lists the number of carbon atoms in the ring, followed by a dash and the word “crown,” followed by another dash and the number of ether linkages (or oxygen atoms). Thus 8-crown-4 is the crown ether having the chemical formula, C8H16O4.
Appendages and other ring constituents can be included in the simplified names, such as benzo-18-crown-6, in which two carbons each of two benzene rings constitute part of the ring perimeter, or diaza-18-crown-6 which has -NH- groups replacing two of the oxygen atoms, -O-.
Molecular Mode of Action
Modified crown ethers can contain nitrogen atoms and additional features. A crown ether molecule of appropriately-sized ring opening has spaced electron-rich oxygen atoms that ensnare a “cation” or positively-charged particle. The cation is water-soluble (it can dissolve in water) but when encased in the crown ether ring, can be “pulled” into other solvents as well. The cation is often an alkali metal ion, such as sodium, potassium, or lithium, or it can be an alkaline earth metal ion, such as calcium or magnesium.
It is also, commonly, an ammonium ion. The size of the opening in the ring is matched to the size of the ion for best effect. Thus, 12-crown-4 works as the choice for lithium, while the larger diameter 15-crown-5 and 18-crown-6 rings work best for sodium and potassium, respectively. The crown ethers used to encapsulate calcium and magnesium frequently include nitrogen atoms.
The ion-containing crown ethers are used in phase-transfer catalysis (see references). This process puts the encapsulated cation to work in a solvent in which it would not ordinarily be found. Another use of cation-containing crown ethers is in cationic exchange polymers. A cationic exchange polymer is the same as a cationic exchange resin: An insoluble (will not dissolve) organic polymer (molecule made of many subunits) having negatively charged radicals (molecules with unpaired electrons or an ‘open’ electron shell) attached to it that can attract and hold positively charged ions (cations) in a surrounding solution.
One such resin listed in scientific journals is di-tert-butyl-cyclohexano-18-crown-6, which is being used in radioactive strontium-90 urinalysis determinations – a test to check for radioactive isotopes.
Another use in the literature, even more complex, is the development of crown ether-modified clays in the formation of polystyrene nanocomposites. Nanometer-sized particles of polystyrene plastic combine with other different materials, to create these nanocomposites.
Crown Ether Modifications
One interesting modification of the crown ether has been developed, with two ringed chemical structures featuring ether linkages, attached to each other via a nitrogen linkage. One of the rings can partly attach to the cation. The application of light then causes the nitrogen linkage to alter, causing the other ring to close in on the other part of the cation. This has been likened to catching a ball (the ion) in a baseball glove (the ether structure). Various sized ions can be “caught” by such a structure.
Crown Ether: A Designer Molecule With a Crown-like Appearance
Crown ethers, named for their crown-like appearance, have many uses, and can be modified in many ways. These designer molecules are vital in detecting Strontium-90 radiation in people who deal with radioactive materials, as well as creating composite materials that mix at the nano-level. Truly, these are fascinating molecules.
Note: You might also enjoy Antibiotics from Dirt?
References:
- Narayan, Ramani: Application of Crown Ethers as Phase Transfer Catalysts in the Electron Transfer Reactions of Coal. Purdue University
- Pedersen, C.: The Discovery of Crown Ethers. (1987). Nobel Prize
- Org-Chem: Molecular Crowns – Crown Ethers
- Yao, H.: Crown ether-modified clays and their polystyrene nanocomposites. (2002). Society of Plastics Engineers

Never ever heard of those before!