Skunk Stench
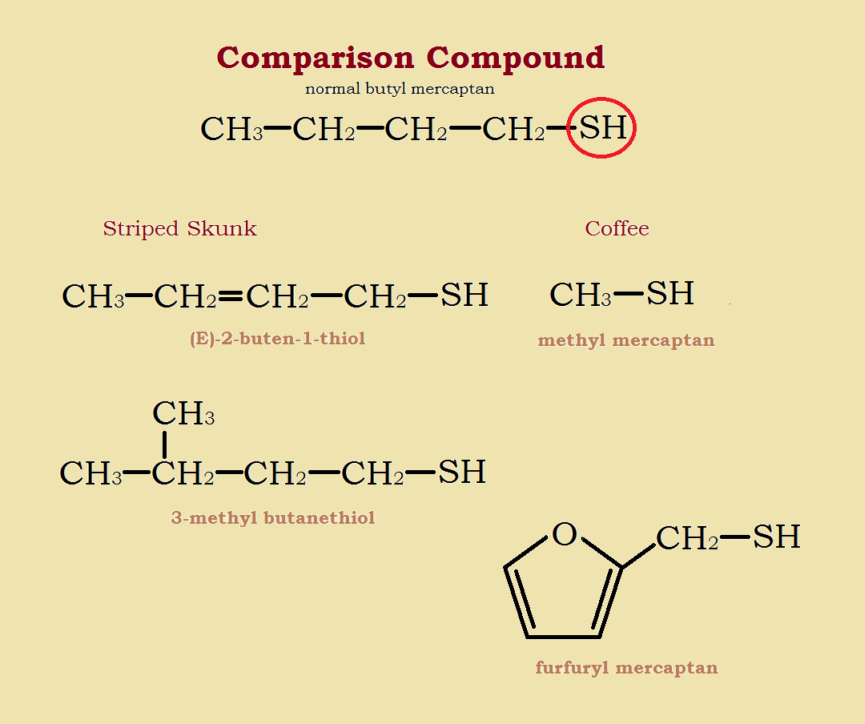
The chemicals that primarily give skunk spray its stench are thiol derivatives—in particular, derivatives of the sulfur alcohol n-butyl mercaptan (CH3-CH2-CH2-CH2-SH).
The portion of this compound responsible for its smell is the -SH group, a sulfur atom bonded to a hydrogen atom, which is similar to the -OH or alcohol group. Common derivatives modify the carbon chain. Another variation chemically modifies the -SH group, using acetic acid (CH3-COOH) to form the corresponding thioacetate.
Two of the most odoriferous skunk spray mercaptans are (E)-2-buten-1-thiol and 3-methyl-butanethiol. Other than its prevalence (it makes up approximately 2/5 of skunk spray) a factor that could intensify its aroma is its double bond, which makes it an alkene mercaptan. The basic carbon chain is not CH3-CH2-CH2-CH2–, but CH3-CH=CH-CH2–. Similarly, the brown marmorated stinkbug produces two foul smelling compounds; both are aldehydes and alkenes.
Coffee Aroma
There are two mercaptans (thiols) especially responsible for coffee aroma—methyl mercaptan (CH₃-SH) and furfuryl mercaptan (C4H3O-CH2-SH). Typically, the smaller a mercaptan molecule is, the more volatile it is, and the more intense its aroma is likely to be. However, these mercaptans are present in very small amounts in coffee. It takes a gas chromatograph to detect them!
In Conclusion
So when is it that the presence of a skunk is likely to smell most like our delicious coffee aroma? It is long after the initial presence of our striped or spotted friend—when its mercaptan levels have diminished, causing its odor to have faded considerably.
Note: You might also enjoy Why Dogs Pee on Tires
References:
- Chemistry of Skunk Spray
- Alarm Odor Compounds of the Brown Marmorated Stink Bug…
- Flavor Scientist: Coffee and Furfuryl mercaptan FEMA 2493