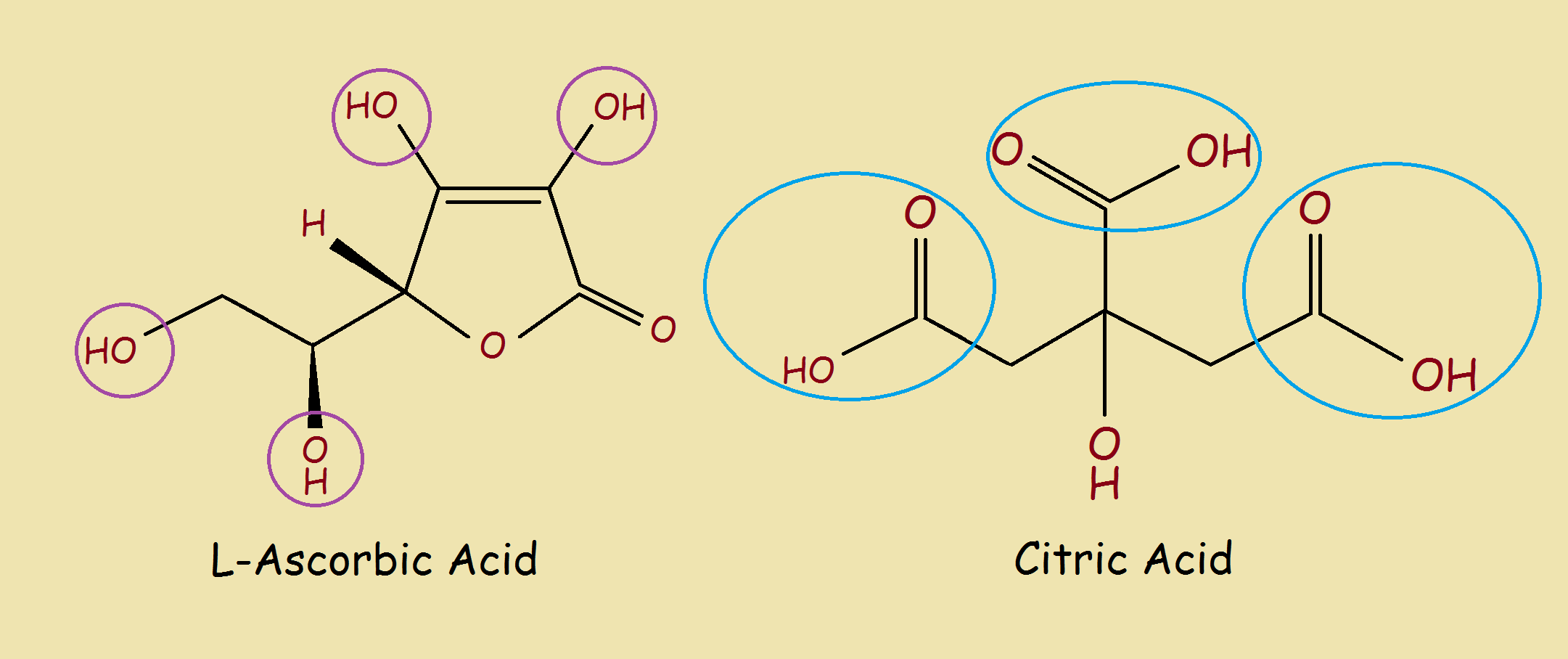
 Two familiar acids, found in citrus fruit, are citric acid and ascorbic acid (Vitamin C). But what are these acids and how are they different? First, consider the structure of these two food acids.
Two familiar acids, found in citrus fruit, are citric acid and ascorbic acid (Vitamin C). But what are these acids and how are they different? First, consider the structure of these two food acids.
Examine the Image
The two acids look different, yet somehow they look similar. Each has a ring, or what almost passes as a ring, and each contains an abundance of oxygen atoms (O). There are three carboxylic acid groups –C=O(OH) are found in the citric acid molecule. On the other hand, ascorbic acid contains no carboxylic acid groups. Yet it, too, is acidic, about as acidic as vinegar.
Citric acid with its carboxylic acid groups is the more acidic acid and so is more sour to the taste.
But why is ascorbic acid acidic if it has no carboxylic groups? It is because it readily parts with a proton (H+), to leave behind what we call a conjugate base. We write (in symbolism),
H–B → H+ + B–
where ascorbic acid is symbolized as H–B, H+ is the proton, and B– is the ascorbate ion conjugate base.
Which Citric and Ascorbic Hydrogens are Favored?
For citric acid, the favored hydrogens are those that are part of a carboylic acid group.
For ascorbic acid, the protons that one might guess could each leave come from hydroxyl groups (–OH), circled in purple. Why is it only the hydrogen atoms on the hydroxyl groups that can leave? Oxygen readily accepts additional negative charge. Carbon does not.1
Ascorbic acid contains 4 such hydroxyl groups. However, not all 4 hydrogen atoms are equal in ability to “leave” their respective oxygens. Why? It has to do with what would be left after a hydrogen leaves. It has to do with stabilization due to resonance.
Since ‘a picture is worth a thousand words’, consider the following Khan Academy video that considers two structures simpler than ascorbic acid, that will help us understand resonance stabilization before we discuss the more complex ascorbic acid…
So the stronger an acid is, the more stable is its conjugate base. To turn that around, the more stable the conjugate base, the stronger the acid.
We’re now ready to consider ascorbic acid.
Focusing on Ascorbic Acid
No resonance structures can be drawn for the isolated hydroxyl groups. Removing their hydrogen atoms would not allow resonance stabilization. But there is resonance stabilization for the two protons shown in the image below.
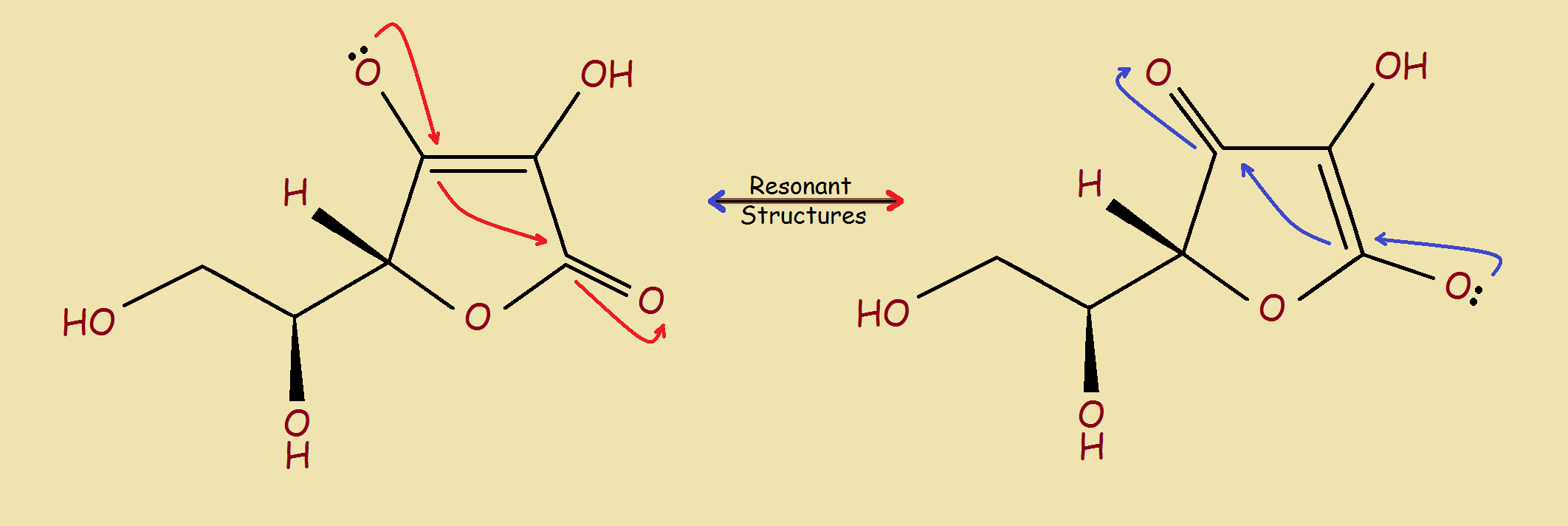
Sodium ascorbate is commonly encountered in food products. A sodium ion replaces one hydrogen atom of the ascorbic acid. We now know which hydrogen atoms do not participate in its formation. We also know why ascorbic acid, although not as acidic as citric acid, is nevertheless somewhat sour.
1 There are instances when a carbon atom carries an extra electron. Such a structure is called a carbanion.
Note: You might also enjoy Chopping Onions Makes You Cry
References:
- Journal of Biological Chemistry: Metabolic Interrelationships of Ascorbic and Citric Acids
- Orthomolecular.org: Vitamin C and Acidity
