 One online definition of chromophore is “an atom or group whose presence is responsible for the color of a compound.”
One online definition of chromophore is “an atom or group whose presence is responsible for the color of a compound.”
Although one may think of a chromophore that includes a metal atom such as copper, nickel, or cobalt, in organic chemistry a chromophore is more likely to consist of a collection of carbon-carbon multiple bonds, perhaps with modifying features.
It is the organic variety we discuss in this article.
Multiple Bonds
Most organic compounds incorporate one or more of three carbon-to-carbon bond varieties: single bonds, double bonds, triple bonds. When drawing a basic organic compound, these are usually represented by: C–C, C=C, and C≡C, respectively.
Although such notations are quite useful, they afford little information concerning bond nature and behavior.
Bond Hybridization
The best working hypothesis for bonding between atoms is one that involves the mathematical combining of atomic orbitals. The most famous of these is doubtless the LCAO-MO hypothesis. LCAO-MO stands for the theory that linear combinations of atomic orbitals produce molecular orbitals, which unite molecules.
The Single or Sigma Bond
A single or sigma molecular bond (σ) is the bonding of two atoms in head-on fashion. It is the strongest variety of covalent¹ bond. Imagine a dumbbell a weightlifter presses overhead. A sigma bond maintains considerable freedom of motion.
The Pi Bond
The double bond consists of both a sigma and a pi bond joining the same atoms. The pi part of the double bond, rather than assuming a head-on configuration, lies above and below the two atoms.
Visualize a pi-bond as a hot dog with two half buns, one half above, one below the meat. The double bond is less mobile than a single bond.
The Triple Bond
A triple bond consists of a sigma bond plus two pi bonds, all associated with the same atoms. It’s something like a hot dog with four half buns spaced out around the meat. A triple bond is relatively rigid.
Colored Organic Compounds and Their Chromophores
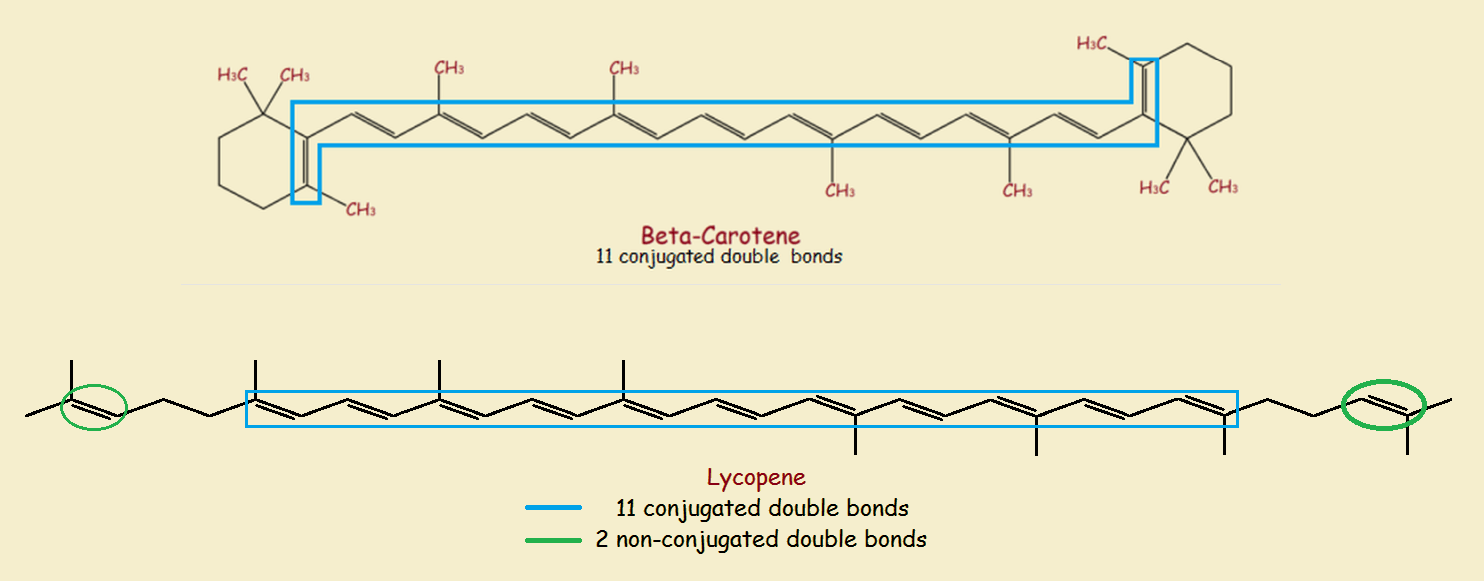
Some organic compounds have conjugated pi systems. That means they possess a series of alternating pi bonds. The image provides some examples.
Sufficiently large conjugated systems of pi-bonds absorb visible light. The light not absorbed, which is the light we see, is no longer white. Thus beta-carotene absorbs blue light, imparting the distinctive orange color of carrots.
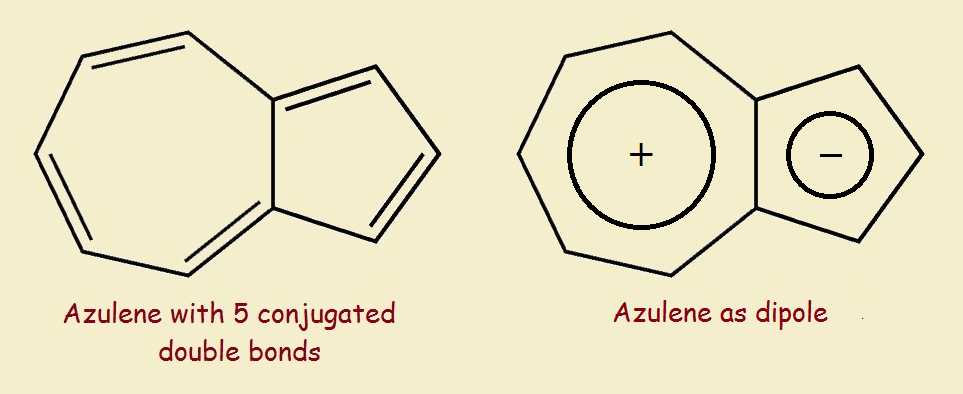
Azulene’s Modifying Feature
Additional multiple bonds that are not part of a molecule’s conjugated pi-system, as well as the geometry of the molecule, may affect color. Thus lycopene is red, rather than orange, despite having 11 conjugated double bonds as beta-carotene does. But some molecules possess strong modifying features affecting color.
Azulene is such a molecule. Azulene, with only 5 conjugated double bonds, is indigo blue. What modifying feature is responsible for this? Consider the two rings of azulene individually.
One ring is a cycloheptatriene ring. The other is a cyclopentadiene ring. If we take one electron from the first ring and give it to the other ring, we wind up with a cycloheptatrienyl cation and a cyclopentadienyl anion.² This dichotomy means azulene possesses an important polarity that affects its chromophoric behavior.
Extra Credit:
If you’ve considered the above and feel comfortable with it, you might want to tackle something with a little deeper. The following Khan Academy video is very informative and is not terribly long (9:33).
The speaker talks rather rapidly, but he does an excellent job of providing insight into multiple-bond, conjugated pi-systems.
1 Rarely, there are other bond varieties, including ionic bonds.
2 See the article Azulene or Cycloheptatrienyl Cation Cyclopentadienyl Anion “Salt”?
Note: You might also enjoy How Does Bleach Bleach? What Removes the Color?
← Back to Classic Science
← Home
