 What is capillary action? The easiest way to define it is to give the simplest example of it at work. A capillary is a tube with a fine bore, typically less than a millimeter.
What is capillary action? The easiest way to define it is to give the simplest example of it at work. A capillary is a tube with a fine bore, typically less than a millimeter.
For the purpose of our discussion, we will use a scientist’s glass capillary tube, which is both straight and clear. The liquids we will discuss as examples are water and mercury.
Not All Liquids Exhibit Capillary Action
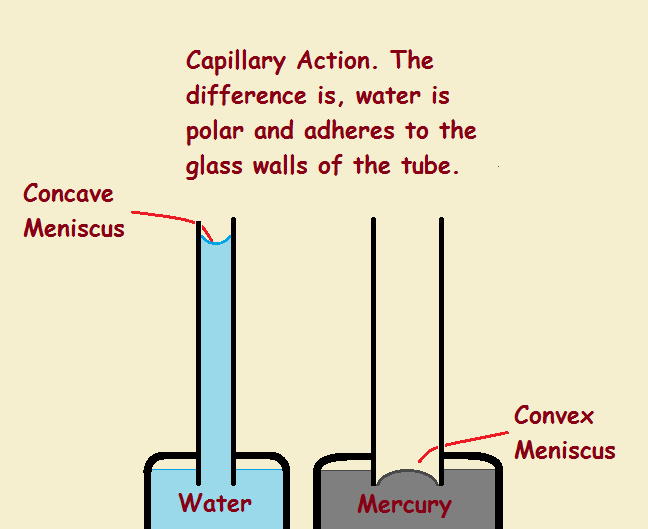
Take note of Figure 1. Two capillary tubes (not drawn to scale) are immersed in liquid – the left tube in water, the right in mercury. The water rises up its tube and forms a concave meniscus at top. The mercury does not rise up its tube. It forms what looks like the upper portion of a sphere – a convex meniscus. Three forces are responsible for the behavior of the liquids – adhesion, cohesion, and gravity.
Adhesion
Water adheres to the glass walls even when there is no body of liquid to support it.¹ This is because it forms hydrogen bonds with the glass, since glass is constructed from oxygen-rich polar silicon dioxide SiO₂ molecules. In fact, water molecules bond more tightly to glass than they do to each other.
That’s not the case with mercury. It is just the opposite. Mercury does not adhere to glass. It fills the tube to just below the level of the supply with a slightly convex shape in the middle of the tube. Nevertheless, one force both water and mercury share in common is cohesion.
A very detailed atomic-level discussion that explains why water adhesion to glass is greater even than cohesion of water molecules and is the controlling factor in capillary action, is skillfully explained in this Khan Academy Video…
Cohesion
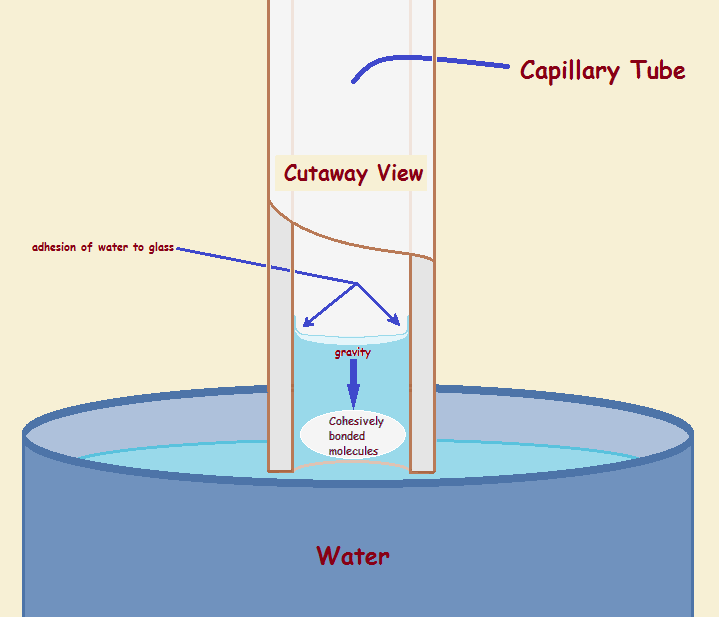
While we have alluded to the way water adheres to glass and forms a concave meniscus, we have not discussed why water rises up the tube to form an elevated column above the level of the supply vessel. This forces us to recognize a second force at work – cohesion. Interestingly, the thinner the tube, the greater is the ratio of the force of adhesion to cohesion and gravity. The thinner the tube is, the less gravity can counter these forces.
An Illustration
To get a better understanding of cohesion, consider a colony of ants. Ants travel across a forest floor. Sometimes they may create an ant “bridge” to cross a gap. The bridge is constructed, the majority cross over, the bridge is disassembled.
The best bridge is a straight bridge, not a sagging bridge. To achieve this, the ants must pull together. It is similar for water molecules. They pull together. Water accomplishes this via hydrogen bonding. These bonds are weaker than ordinary chemical bonds, yet they behave much like weak springs pulling the molecules together and up (thus successfully countering gravity).
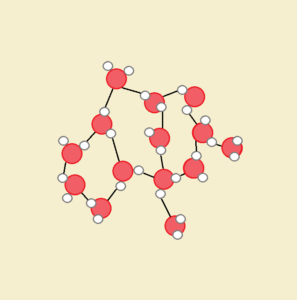
Figure 3 pictures the hydrogen bond connections between water molecules. The hydrogen bonds counter part of the gravity exerted on the water in the tube, much as the ant bridge resists the weight of the ants crossing over it.
Hydrogen Bonds
As discussed at length in the article The Dipolar Molecule Water, water (H₂O) is not a straight molecule, but is shaped like a mouse’s head. Its H-O-H structure is bent. The positive charge of the hydrogen atoms is concentrated on one side of the lone oxygen atom, while the other side of the oxygen maintains a slight negative charge. Water atoms are thus polar. That means they behave much as a magnet does, with its north and south poles.
Water molecules attract each other, as well as any other nearby charged atoms or molecules. This attraction or pulling together is the source of water’s cohesive character. See Figure 2. While mercury liquid exhibits cohesion for other mercury atoms, it does not have a net charge, nor does it form hydrogen bonds. Hence, mercury atoms do not adhere to glass. Mercury does not exhibit capillary action.
I Think I Shall Never See
The song goes, “I think I shall never see, a poem lovely as a tree.” A tree contains tiny capillary tubes that transport moisture and nutrients. Assisting the process is the input of water via the roots, and the transpiration of moisture through the leaves. It might be suggested that capillary action is the heart and life of the tree. Certainly there can be no more regal example of this than that of the California Redwood.
¹ See, for instance, Beer Bottle Condensation: What Forces Produce Droplets?
← Back to Classic Science
← Home
