 Dan asked us¹…
Dan asked us¹…
“My question concerns condensation droplets. What dictates how droplets form, then combine with each other? When you blow warm breath onto a cool surface, at first nothing appears to happen. Keep it up and droplets appear. These small droplets merge into bigger droplets. What physical laws dictate how this occurs? Also, what role does gravity play on vertical surfaces such as a chilled bottle of beer, producing tiny rivulets of moisture running down the sides?”
Initial Commentary
The answer, which follows, although it has some basis in well-known physical principles, depends in part upon observation, mental visualization, and (finally) blatant speculation.
This is an interesting procedure, since so many of life’s mundane occurrences are in reality quite fascinating when closely examined.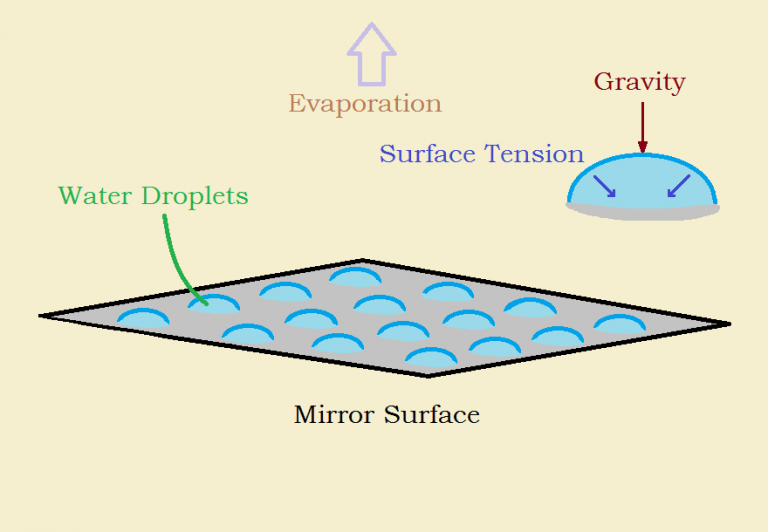
Initial Condensation
We breathe in and breathe out, exhaling water vapor at a temperature of approximately 34°C (93.2°F). We may exhale onto a mirror in order to wipe it clean with a tissue.
Actually, a mirror is excellent for discussion purposes. The glass of most mirrors is formulated from silicon dioxide. Fused silicon dioxide is polar,
and thus glass is polar. Since water is also polar, it readily adheres to glass. No scratches or other surface imperfections are necessary.
What is polarity? It is an uneven distribution of molecular electric charges. (One end is positive, one end is negative.) Ends of molecules with opposite charges attach themselves to one another.
Simply put, condensation droplets adhere by electrical charges.
Opposing Forces at Work
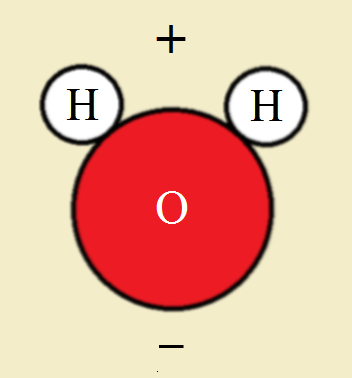
Our breath deposits tiny drops of moisture on a mirror. However, any moisture already present on a mirror’s surface tends to evaporate due to vapor pressure. If the relative humidity of the air at the mirror’s surface is sufficiently high and is constant, we will build up a foggy film. But if we stop exhaling and the humidity is low, condensation droplets quickly evaporate.
What enables continuous bombardment of the mirror’s surface with moist air to eventually build up droplets of moisture? As moisture molecules continue to bombard the mirror, some of them strike where there already are adherent water molecules. Hydrogen bonding causes not only the water molecules to stick to the silica of the glass, but the newcomers to bond to those water molecules already present. As the droplet radius increases in size, other physics factors play an increasing role.
The Other Factors
Two of those other factors are gravity and surface tension. Now we are all acquainted with gravity, but what precisely is surface tension? The Oxford Dictionary tells us that it is,

“The tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid, which tends to minimize surface area.”
The particles mentioned in the above definition are polar, that is electrically charged, molecules of water. Those particles within a drop of condensation all thoroughly bond to one another. But the outermost layer has no particles above to bond to, so those particles bond to the particles below with even greater fervor, forcing the drop into its somewhat spherical shape.
The vessel of minimum surface area that contains the maximum volume is a sphere. It would seem logical then that condensation would resemble somewhat flattened hemispheres.
As the radius of each drop increases, so does the pull of gravity on each droplet, spreading the droplet out. Surface tension pulls the droplet up and together, so it does not lose its spherical shape too rapidly.
Eventually, if the source of additional moisture continues at a sufficient rate, gravity will win out and the drops will become rivulets or sheets of moisture. This is especially so on a well-cooled vertical surface such as a beer bottle.
The difference in the case of the beer bottle is that its chilled surface condenses a sizeable percentage of the water vapor in the atmosphere around it, no matter what the relative humidity may be.
Physics, Water, and Condensation
Observing the behavior of water condensing on the surface of glass is science you can experience at home. For more information about surface tension, cohesion, adhesion, and more, please check the resources for this article.
1 First addressed on the website Decoded Science, this article was categorized under physics and philosophy. Why philosophy? When one is relaxed, sipping on a beer, they tend to put on the hat of the philosopher, reflecting upon the insignificant.
Note: You might also enjoy Massless Strings and Frictionless Pulleys
References:
- Khan Academy. Cohesion and Adhesion of Water
- Institute for Microelectronics. Silicon Dioxide Properties
- DUI Answer. Breath Temperature: An Alabama Perspective
- Oxford English Dictionary. surface tension. (2017)

When I was a child, we had no computers or mobile phones and when we visited my grandmother, we had no TV either! (I don’t have one now, but that’s by choice.) If it rained, the only indoor entertainment we had were dominoes or coloring pages. Sometimes, we didn’t even have those, so it was fun to watch the raindrops slide down the windowpane. I don’t know if modern day kids would consider watching raindrops on glass as “fun” but it teaches you a lot about drop size, speed, etc.