 A banana bond is not a typical chemical bond by any stretch of the imagination. It is a 3-center, 2-electron bond. The shape of this kind of bond resembles a banana, hence its name. Perhaps the simplest example of a banana bond is demonstrated between boron and hydrogen in the diborane molecule, B2H6.
A banana bond is not a typical chemical bond by any stretch of the imagination. It is a 3-center, 2-electron bond. The shape of this kind of bond resembles a banana, hence its name. Perhaps the simplest example of a banana bond is demonstrated between boron and hydrogen in the diborane molecule, B2H6.
Elemental Atomic Orbitals
We begin with a discussion of the much simpler, more typical 2-center, 2-electron single bond. When atoms form molecules, the atomic orbitals involved transform into molecular orbitals.
Let’s consider a very simple example. Say we want to form one C-H bond of the molecule methane (CH4). Now hydrogen atoms only have one electron. The single electron lies in the 1s2 orbital. That type of orbital possesses spherical symmetry.
Unlike hydrogen, carbon has 12 electrons total. Its electron layout is
1s22s22p2
The number represents electron shells. The first shell can only hold two electrons, so it is filled. Those two electrons will not participate in the formation of a molecular bond. The second shell consists of two subshells, s and p.
The 2s subshell is filled, but the shell is not filled, since the 2p subshell can hold 6 electrons in 3 orbitals. These are called the 2px, 2py, and 2pz orbitals.
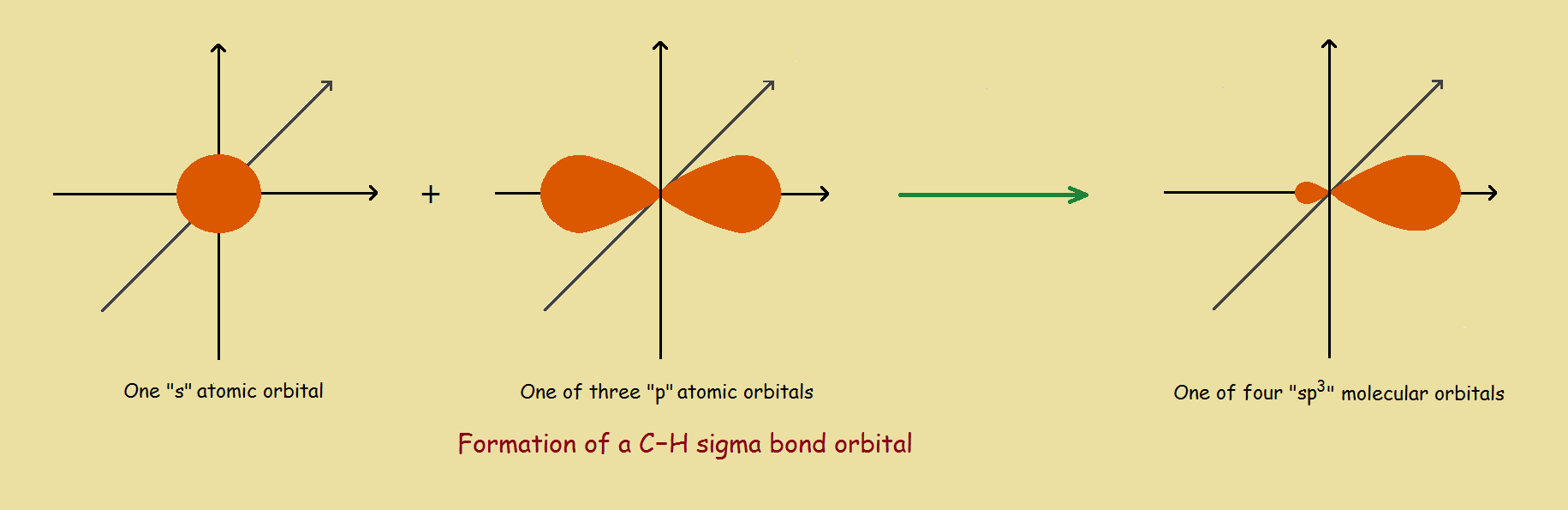
Energy is the controlling factor. Carbon ordinarily exhibits a valence of 4. So 4 atomic orbitals can be utilized to form 4 molecular orbitals. However, all 4 bonds are equivalent. So the one 2s and the three 2p orbitals yield their electrons to produce [at some point] 4 sp3 molecular orbitals.
Our hydrogen atom contributed an electron in its 1s orbital to the overlap process with one of the carbon sp3 orbitals, to yield a C-H single bond. This head-to-head bond is classified a sigma (σ) bond.¹
Let’s Move On to a 3-Center, 2-Electron or Banana Bond
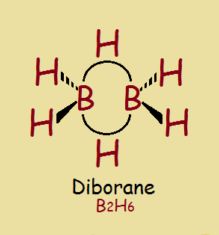
When we thing in terms of atomic orbitals, why do two of the hydrogen atoms in diborane behave atypically? Well, let’s examine them in terms of their composite atomic orbitals.
The electron structure for boron is
1s2 2s2 2p1
This suggests boron ordinarily has a valence of 3 from its 2s and its 2p electrons, 2 + 1 = 3. Yet in diborane, we see what looks to be a valence of 4!
The following Oxford Academic video aptly describes what is going on for the diborane molecule, B2H6. It is short, brief, clear.
1 The single bond consists solely of this sigma bond. Carbon can also form double and triple bonds. A double bond consists of the sigma bond plus a pi (π) bond, which we do not discuss here. A triple bond consists of the sigma bond plus two pi bonds.
Note: You might also enjoy Organic Chemistry: Pushing Electrons
← Back to Quirky Nuggets
← Home

Thanks for finding that video, good clear explanation.