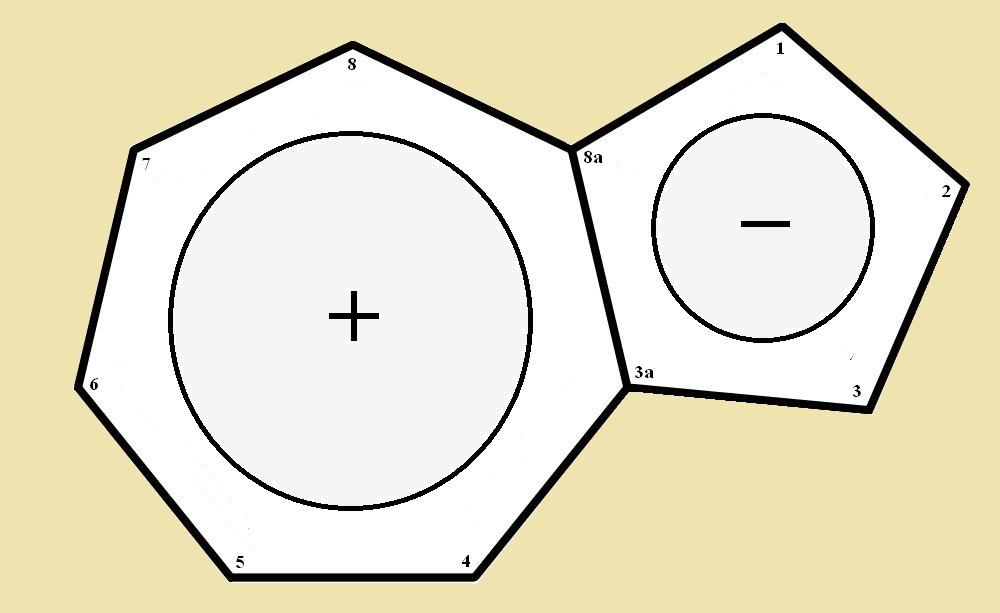
Azulene is a fascinating hydrocarbon. It is bicyclic, meaning it has a kind of double ring structure. It is a seven-member ring adjoined to a five-member ring by a shared two-carbon wall. One ring resembles cycloheptatriene. The other ring, cyclopentadiene. And yet, there is something different about azulene. Or should we say some things different?
Unusual Qualities
Although azulene is a hydrocarbon, it is a deep blue hydrocarbon, something very much out of the ordinary. The Lactarius indigo mushroom, in fact, gets its blue coloration from a derivative of azulene. In addition, although most plain hydrocarbons have little or no dipole moment, azulene has a dipole moment of 1.08 Debye units. This is equal to that of the nitrogen-containing diphenylamine molecule.
One ring possesses a very definite positive (cationic) character. The other, an equivalent negative (anionic) character. And though both neutral rings would be in themselves, non-aromatic, the two in combination are both aromatic. At least they possess aromatic character.
Azulene Resembles Tropylium and Cyclopentadienyl Ions
The seven-member ring is cationic, and as such is analogous to the cycloheptatrienyl cation, a.k.a. the tropylium ion. The five-member ring is anionic and corresponds to the cyclopentadienyl cation. Both of the ionic rings, taken separately, are aromatic. The combination, as was pointed out, possesses aromatic character. In a sense, azulene resembles a salt undissolved, with a bound positive and negative portions.
The Wall Makes the Difference
The “wall” they share in common is what provides the opportunity of aromaticity. That prevents azulene from being a monocycle. If the carbon atoms at 3a and 8a were split apart and their bonds to each other satisfied with the addition of two hydrogen atoms, one would have the molecule cyclodecapentaene. This at first would seem it should be aromatic, but the combination of angle strain and steric strain forbid it.
The title of this article made an almost humorous reference to azulene’s being a kind of salt. While that is not true, an undissolved salt does have a positive portion or cation attached to a negative portion or anion. The analogy is real.
Note: You might also enjoy Cyclopropenone Aromatic or Not?
References:
← Back to Classic Science
← Home