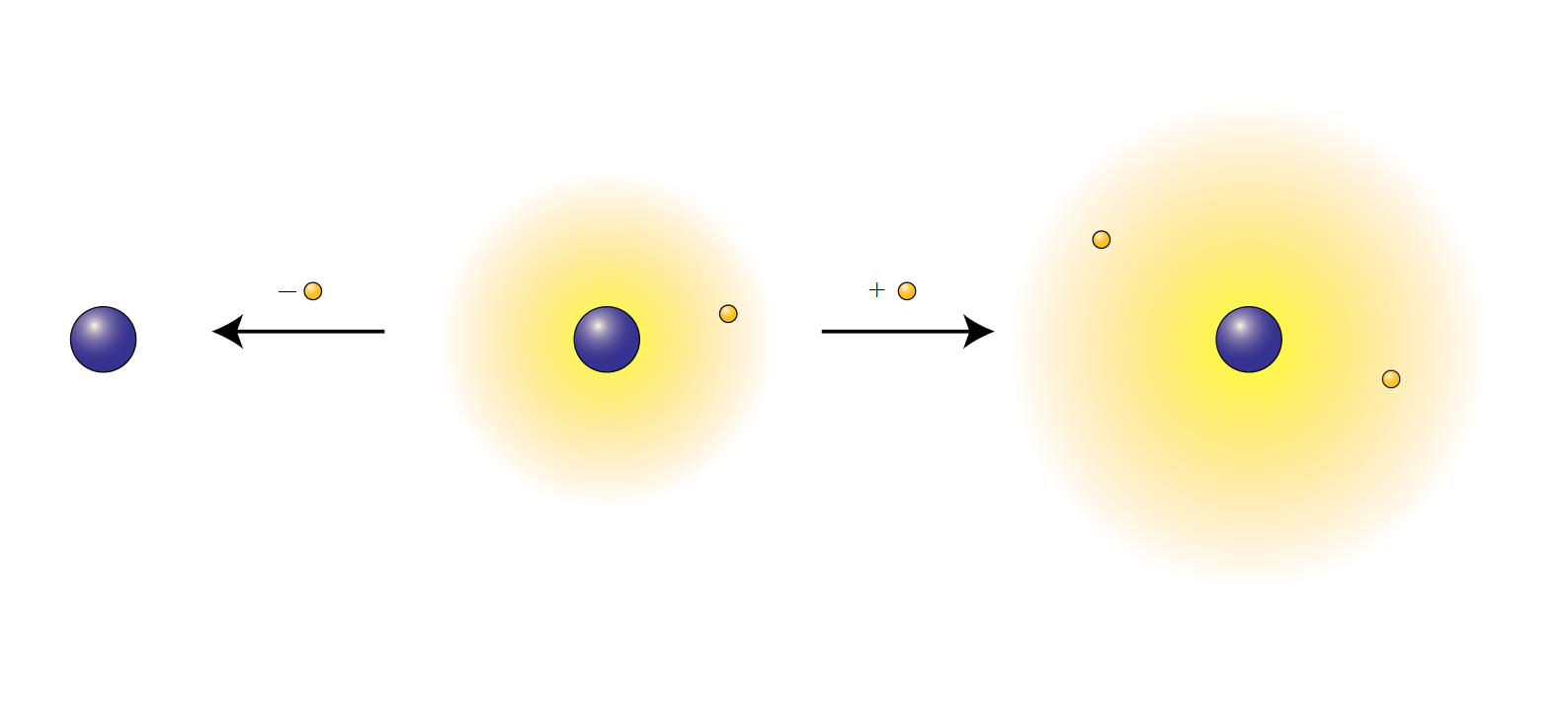
Not all atoms are atoms that are ionized.
All atoms have a nucleus containing protons and neutrons. That nucleus is surrounded by one or more orbitals that contain electrons. The total charge of a neutral atom equals zero. The number of protons equals the number of electrons. If the number of electrons increases or decreases, an atom is ionized. It is either a cation (positive ion) or an anion (negative ion).
Atoms That Are Ionized
The same thing can happen to small collections of bonded atoms. By definition, an atom oxidizes to a cation or reduces to an anion.
Positively Charged Atoms (Cations)
Atoms that are ionized have lost energy and become more stable in so doing. Some atoms such as sodium (Na) are most stable if one electron is lost. The sodium atom has 11 protons and 11 electrons, but the cation has only 10 electrons. The reaction for ionizing sodium is written Na → Na⁺ [plus an electron, e⁻].
Other atoms lose 2, 3, 4 or more electrons. Calcium loses two electrons to form Ca+2; aluminum loses 3 electrons giving Al+3. Ruthenium loses 4 electrons to give Ru+4; uranium parts with 5 electrons, leaving U+5. Selenium yields Se+6. Manganese forms Mn+7. Osmium loses 8 electrons to form the highest oxidation level, Os+8.
Complex Cations
Sometimes a collection of bonded atoms loses one or more electrons. Occasionally, instead, it may gain a hydrogen cation, H+.
The uranyl cation (UO2)+ consists of one atom of uranium bonded to two oxygen atoms. Chromyl is the combination of a chromium atom and two oxygen atoms resulting in a divalent, or double-charged cation, (CrO2)+2.
Ammonia, NH₃, accepts a hydrogen cation to become an ammonium cation, NH4+. Likewise, phosphine, PH₃, accepts a hydrogen cation, giving phosphonium, PH₄⁺.
Negatively Charged Atoms (Anions)
Some atoms such as fluorine ionize by gaining electrons; F → F–. As with the atoms that form cations, other atoms can form anions with multiple charge. Oxygen, sulfur, selenium and other atoms can gain two electrons. Nitrogen, phosphorous and arsenic can gain 3 electrons. More highly charged anions are generally found among the complex anions.
Complex Anions
Many negatively ionized atoms form complex anions. Single-charged complex anions include nitrate, NO3, cyanide, CN, hypochlorite, OCl and permanganate, MnO4. Anions with a –2 charge include sulfate, SO4, carbonate, CO3 and dichromate, Cr2O7. Examples of ions with a –3 charge are phosphate, PO4, aluminate, AlO3 and silicate, SiO4. Some complex anions have a –4 charge, such as stannate, SnO4, antimonate, SbO4 or ferrocyanide, Fe(CN)6.
Note: You might also enjoy Introduction to Chemistry Subscripts and Superscripts
← Back to Classic Science
← Home

Thank you for sharing this article.