In Part One of The Quintessential Aromatic Hydrocarbon Benzene, we saw that the so-called 1,3,5-cyclohexatriene was really not completely described by the structure that that name implies. It is equivalent to 2,4,6-cyclohexatriene (if you flip the 1,3,5- structure over, and you get the 2,4,6- structure), and includes a variety of other contributing factors. Although we did not list them, they may include ionic contributors.1 In general, these are discounted.
Visualizing Benzene’s Structure
The conclusion is that the compound actually has six equivalent bonds between adjacent carbon atoms, forming six internal angles angle each equal to 60°. The bonds are each, then, “single-and-one-half” bonds between each carbon pair. The molecule is flat, even as a collection of hexagonal bee honeycomb chambers are flat. This allows something very special to happen in the chemistry of benzene.
Before we speak of that, let’s discuss varieties of carbon-to-carbon bonds. Single bonds are called sigma bonds. In -C-C- the central dash (-) represents the sigma bond. As the sketch suggests, that bond lies directly between the two carbon atoms.
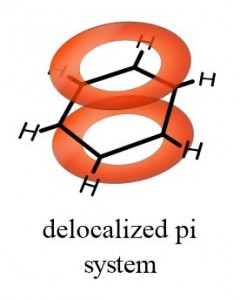
Now things are not so simple for double bonds. Although they are written as if they have both bonds lying between the connective atoms (-C=C-), this is misleading! The second bond actually lies above and below the atoms, rather than between them. Notice Figure 1.
Pi-Bonds: Something Special
The one bond, which is both above and below the plane of the molecule, is called a π (“pi”) bond. If a magnetic field is applied perpendicular to the plane of the molecule, this inductively stimulates the pi bonds of benzene to generate a “ring current.” This phenomenon is illustrated in Figure 2.
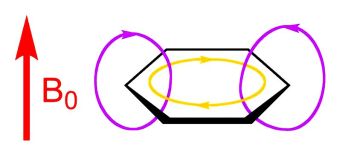
- Fig. 2 – Induced Aromatic Ring Current: current color-coded gold.
The resonance, discussed earlier, results in a lowering—or stabilization—of energy. The benzene molecule is more stable than any of its contributing resonance structures. It also affects the chemistry—which will be discussed in the concluding part of this article, Part Three.
Concluding Comments
We have seen illustrated in the case of benzene, the properties that define aromaticity in an organic compound, namely, these properties:
First, the molecule or species must be cyclic.
Second, the molecule or species must contain completely conjugated double bonds for the portion of the molecule that is aromatic.
Third, the molecule or species must be flat—or at least very close to flat—to enable the appropriate π-bonds to establish a potential ring current.
There is an additional requirement that will serve as part of a separate article: that the number of conjugated pi-electrons should equal the sum of 4n + 2, where n is an integer up to approximately 6. On all counts, benzene possesses an aromatic structure.
1 An ionic contributor means a structure that includes a partial positive and an equivalent partial negative electrical charge within the same molecule.
[End of Part Two] Proceed to Part Three
References:
- Khan Acdemy: Resonance of Benzene and the Carbonate Ion (video)
- UCLA: Chemistry Tutorial: Aromaticity
- Chemguide: Bonding in Benzene
← Back to Classic Science
← Home
