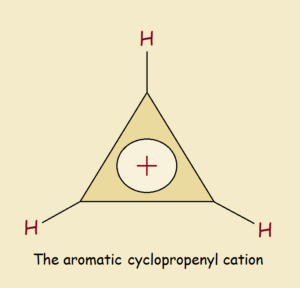
 Can there exist something as small as a 3 carbon atom aromatic cyclopropenyl cation? Let’s find out.
Can there exist something as small as a 3 carbon atom aromatic cyclopropenyl cation? Let’s find out.
The simple Hückel description of aromaticity states a ring shaped molecule will be aromatic if it meets certain criteria. One is the species must be flat. Another is it must contain (for a small integer n) 4n + 2 π-electrons. Mathematics tells us three points define a plane. So a cyclopropenyl ring must be flat!
As to π electrons, when n = 0, the ordinary cyclopropene molecule is not aromatic. It becomes aromatic only if the third π electron is missing. That is, when it becomes the cyclopropenyl cation and its ring has a positive charge.
C3H3+
If delocalization was not a factor, this ion would be drawn as an unequal sided triangle. There would be a somewhat shorter double bond between two of the three C atoms. Each of the three atoms would have an attached hydrogen atom. Only one of those carbon atoms would carry the positive charge.

However, delocalization is a factor. This is due to symmetry. So each of the identical carbon atoms must be connected to its two neighbors by something more than a single bond but less than a double bond. Each carbon atom also bears approximately a +1/3 charge.
It is the Aromatic Cyclopropenyl Cation
The structure is stabilized by the equal division of electrons among the skeletal atoms. It is a kind of average. It is called delocalization. It is as if the double bond moves around the ring at will. It is called resonance. In this case, resonance stabilization is greatly reduced. Why? Due to strain from the abnormally acute angles at each vertex of the carbon atoms.
Note: You might also enjoy Is Cyclopropenone Aromatic or Not?
References:

[How is it that] the compound is aromatic when it doesn’t have conjugate[d] bond[s]?
Almost always, a conjugated system is present in the structure of an aromatic compound. Such is not an absolute requirement, however. If you draw a triangle of carbon atoms and place the plus sign on the top carbon and the double bond at the bottom, it is possible to get a misconception of the cyclopropenyl cation. You see, the double bond could have been drawn between any two of the carbon atoms, as long as you draw the charge on the remaining carbon. Thus a ring current exists. This is the primary determiner. Other aromatic features demonstrated by the cyclopropenyl cation are its flat geometry and the Hückel requirement. The species is aromatic. Never let a rule convince you that a reality is untrue.
Is a diazirine aromatic?
It would appear the unbonded electron pairs on the nitrogen atoms disrupt any such possibility. Aromaticity bears the hallmark of increased molecular stability. Diazirines undergo photolytic reactions. This reference is the closest assist I can provide: http://link.springer.com/article/10.1007%2FBF00527111 Add in the fact that it is the cation of cyclopropene that is aromatic, and not the neutral molecule itself. Could the carbon atom in a diazirine lose electrons and tap into the electron-richness of the nitrogen double bond? Hardly. The nitrogen atoms are electrophilic. Based on these things alone, I would say it might be possible (based on pendant groups on carbon) to come up with an aromatic system, but it would be a difficult task at best. Now toss in the concept that the unbonded electron pairs cannot play a role in connection with the carbon atom, as then the double bond system would not be conjugated.
At last – the article I’ve been waiting years for! Thanks!
Me too, Kasman! I did some university chemistry but this is beyond my realm of competence. Vince, can you remind me – aromatic, does that have the same meaning as in the culinary sense, that is having a strong smell?
Aromatic compounds – many of them – do have a distinctive aroma. It did have something to do with the category name.
The secret of success is to work harder than others every day.
Is it the same with the cyclopropenyl anion?
No. Because then you’d have the pi-electrons = 4 which is a 4(n) and not a 4(n) + 2 multiple.