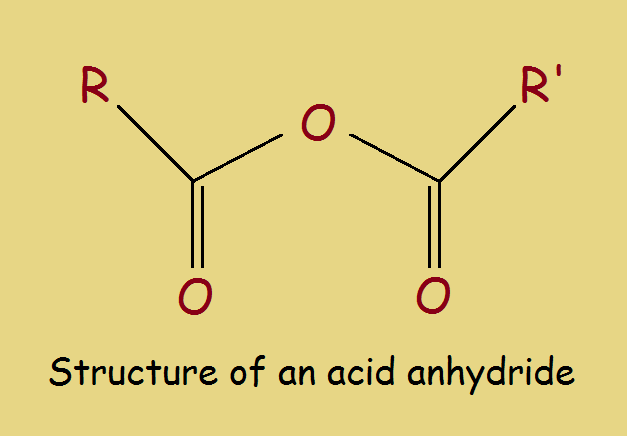
Anhydrides are compounds that are similar to other compounds from which one or more molecules of water has been eliminated.
An anhydride must not be confused with an anhydrous compound. In an anhydrous compound, the water reactant from which it is formed includes water that is attached lightly by weak bonds. Water is not an inherent part of the molecule’s structure.
Consider cupric sulfate pentahydrate, CuSO₄•5H₂O. This is a blue, crystalline substance. It is a composite structure of one molecule of cupric sulfate and five weakly held molecules of water of crystallization. Those water molecules can be removed quite easily.
Powdering the crystals and warming them in a drying oven produces CuSO₄. This anhydrous compound is nearly white. No, it is not an anhydride.
Aluminum Oxide
Aluminum oxide, Al₂O₃, is an inorganic compound and a true anhydride. The hydrated equivalent is aluminum hydroxide, Al(OH)₃. The conversion from the hydrate to the anhydride is written,
2 Al(OH)₃ → Al₂O₃ + 3 H₂O
The reactant on the left side of the arrow offers 2 aluminum atoms. Inside the parentheses, there is listed 1 oxygen and 1 hydrogen. However, the little 3 means you multiply each of those numbers by 3. This makes 3 oxygen atoms and 3 hydrogen atoms. But these 3 each are per molecule and there are two molecules. So there are 6 oxygen atoms and 6 hydrogen atoms on the reactant side of the arrow.
On the right side there are 2 aluminum atoms and 3 oxygen atoms, while there are 3 molecules of water. Each of these possesses 2 atoms of hydrogen and 1 atom of oxygen. This gives a total of 6 hydrogen atoms and 3 oxygen atoms. Adding the atoms from the oxide and from the water together, we get the same numbers of atoms of aluminum, hydrogen, and oxygen as there are to the left of the arrow. The equation balances.
Ethyl Ether
Ethyl ether (AKA diethyl ether) is an organic compound. It is a true anhydride. Its chemical formula is written a number of ways. For simplicity, we write it here as H₅C₂-O-C₂H₅. It is the anhydride of ethyl alcohol (ethanol), C₂H₅OH. The reaction is very simple.
2 C₂H₅OH → H₅C₂-O-C₂H₅ + H₂O
Under ordinary conditions, a dehydrating agent is required.
Acetic Anhydride
The reaction above represents the anhydride of an alcohol. However, organic acids can also be converted into anhydrides. Acetic acid (CH₃COOH) changes into acetic anhydride (CH₃COO-O-OOCCH₃).
Ring Closure Dehydrations
Sometimes an organic dehydration does not involve two molecules, but just one. A ring closure can be the result. Phthalic acid dehydrates to form phthalic anhydride (see the illustration).
Other Anhydrides
There are other reactions that produce anhydrides. The key to identifying an anhydride is that elimination of water must produce a simple, new structure. Sometimes dehydration reactions are called elimination reactions.
Note: You might also enjoy Organic Chemistry: What is a Lactam?
References:
- Mr. Wilson Science: Hydrates – Hydrated and Anhydrous Compounds
- Southwest College: Dehydration of Alcohols – Dehydration of Cyclohexanol
← Back to Classic Science
← Home