Housewives and others running the home are urged not to mix household ammonia plus bleach together. Why? Because the result is a poisonous gas. What gas? What is the chemical reaction that produces it?
Bleach
There are two varieties of household bleach. There is oxygen bleach. And there is chlorine bleach. It is the chlorine variety that causes the risk. Chlorine bleach is a solution of sodium or calcium hypochlorite in water. Its strength runs 3 to 5 percent in water.
Ammonia
100% ammonia is not a liquid. It is a gas. Household “ammonia” is about a 5 percent water solution. Ammonia gas has the formula NH₃. The solution is sometimes written NH₄OH.
NH₃ + HOH → NH₄OH
In reality, most of the ammonia is merely the dissolved gas.
The Dangerous Combination
What happens when ammonia and bleach are combined? Consider the chemistry of the following possibilities:
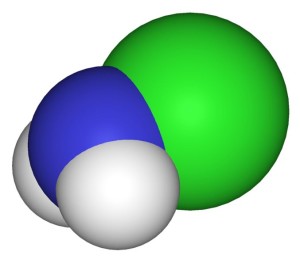
NH₃ + NaOCl → NH₂Cl + NaOH [monochloramine]
NH₃ + 2 NaOCl → NHCl₂ + 2 NaOH [dichloramine]
NH₃ + 3 NaOCl → NCl₃ + 3 NaOH [trichloramine or nitrogen trichloride]
Which of these reactions is the correct one? All three can occur. Which is predominant depends upon “pH, temperature, turbulence, and the chlorine to ammonia ratio.”
Danger to the Householder
Chloramines attack mucous membranes. They irritate the lungs. Inhaled chloramines tie up the hemoglobin of red blood cells. It makes those cells incapable of carrying oxygen. Chloramines are under investigation for the possibility of reproductive effects.
When Organic Matter is Present
In the presence of organic matter, chloramines produce trihalomethanes (THMs). These compounds may be carcinogens. They are of concern in treatment plants, that treat wastewater with chlorine gas or with chloramines. Residents in some cities and towns actively seek to stop the use of chloramines.
Ammonia Plus Bleach Safe? Eh…
The EPA maintains that chloramines, properly used are safe. Still, a homeowner should never mix ammonia with bleach.
Note: You might also enjoy What is the Difference Between Soap and Detergent?
References:
