What are allotropes? An allotrope is one of a variety of forms in which an element can exist. This does not refer to the state of an element, whether solid, liquid, gas, or plasma. It refers to the tendency of an element to exist in different structural forms.
Allotropes of Carbon
Thus carbon can be found in a variety of forms, including graphite, charcoal, diamond, fullerenes, and nanotubes.
Of Sulfur
Sulfur allotropes take an assortment of forms. These include rings that range from six to twenty atoms. Especially well known are the monoclinic and rhombic crystalline forms.
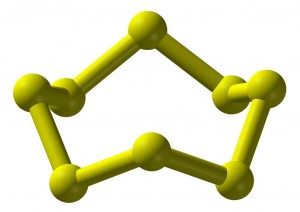
Of Phosphorous
Phosphorus allotropes exist in a variety of colors. Ordinary phosphorous is called white phosphorous (P₄). It is unstable and dangerous. But there are also red, violet, and black forms of phosphorous. These offer greater stability.
Modifying the connections between phosphorous atoms gives other forms of phosphorous. The black form exists as interconnected hexagonal rings. This resembles the graphite form of carbon.
Of Oxygen
Even oxygen assumes a variety of allotropic forms. Ordinary oxygen is a diatomic molecule, O₂. Ozone consists of three oxygen atoms, O₃. Tetraoxygen (O₄) and Octaoxygen (O₈) are also known.
Of Selenium & the Metalloids
There are red, gray, and black forms of selenium. Metalloids exist in a variety of allotropes. Among the elements that form allotropes are boron, silicon, arsenic, germanium, and antimony.
Compounds?
Compounds display properties similar to allotropy. However, they are said to exhibit polymorphism. There are a host of forms of polymorphism (too many to discuss here). All of them are of great interest to the materials scientist.
Note: You might also enjoy Graphene Properties, Applications, and Production
References:
