
Oil and water do not mix!
Some liquids are miscible; that is, they mix completely. Other liquids do not permanently mix. They are immiscible. The best known example of this is oil and water.
“Putting those two together is like mixing oil and water.” This means that for practical purposes, the two don’t get along at all.
Why Don’t They Mix?
Oil and water don’t mix for a basic physical reason curiously easy to explain by comparison with a magnet. Consider first the formula, and then the structure, of water and we’ll see how this is so.
The Formula of Water
Water is most commonly written H₂O. Two hydrogen atoms (H) are combined with one oxygen atom (O). Sometimes water is written H-O-H, as if the three atoms lie in a straight-line, with 180o angles between adjacent atoms. The chemist knows this is not a completely accurate representation, but for most purposes, it doesn’t matter. It is quick and easy to write H-O-H.
The Structure of Water
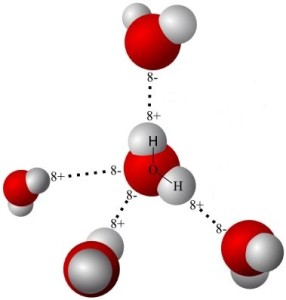
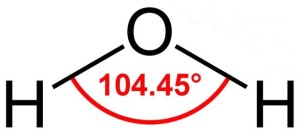
Actually, water molecules are bent considerably. Hydrogen atoms are tiny compared to oxygen atoms, and the two hydrogen atoms in water lie a mere 104.45o apart, on the same side of the oxygen atom. This gives the water molecule a resemblance to Mickey Mouse’s head.
While its size of hydrogen is much smaller than that of oxygen, the electrical charge of two atoms of hydrogen is equal in magnitude (though opposite in sign) to that of one atom of oxygen. So the total charge on a molecule of water is zero. The catch is, the charges are not uniformly distributed.
Polarity and Non-Polarity
Water molecules have a positive side (at the hydrogen end) and a negative side (at the other end). Unevenly distributed electrical charges give rise to polarity in much the same way as a magnet has a north and a south pole. Although there are differing degrees of the characteristic, many molecules are labeled polar or non-polar.
A Kind of Chemical Magnetism
Water is a polar molecule. Oil is a non-polar molecule. Just as the north pole of a magnet attracts the south pole of another magnet, the positive part of one polar molecule attracts the negative part of another polar molecule. Thus water molecules are drawn to each other.
Oil and Water
Oil molecules lack this mechanism. So when oil and water are mixed and violently shaken, both liquids quickly form a multitude of droplets. These may co-exist momentarily. But the water molecules are drawn to each other, so the little droplets of water soon coalesce, leaving the oil behind. Oil and water do not mix.
Note: You might also enjoy: Polymeric Water Clusters
References:
