How do double bonds flip, and what is the significance?
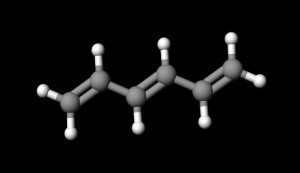
The shorthand drawing of a double bond looks like an equal sign between two atoms. The double bond between the two carbon atoms of ethene gas, H₂C=CH₂, well illustrates this.
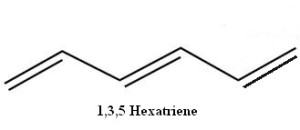
Some organic compounds possess conjugated (alternating) double bonds. A simple example of this is 1,3,5-hexatriene.
What If?
But what if the ends of that 1,3,5 hexatriene are joined, with the loss of two hydrogen atoms, to make a ring one might be tempted to call 1,3,5-cyclohexatriene? In fact, such a molecule, if flipped left-to-right, is seen to be identical with 2,4,6-cyclohexatriene! The numbers can be dropped and the molecule can simply be named cyclohexatriene.
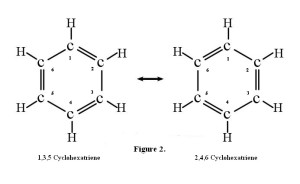
So what can be said? A simple relocation of the double bonds changes the one into the other, yet they are identical. Did the double bonds change location by flipping? In fact, they did not change location at all. There are no single or double bonds at all in cyclohexatriene.
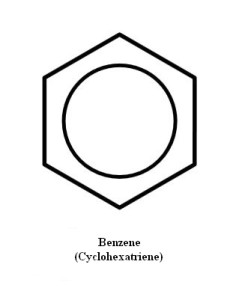
Rather, all the carbon-carbon bonds are of the same length and strength, and are in-between single and double bonds. Thus we can draw this molecule in any number of ways. One convenient method is illustrated in Figure 3. Notice that another name has been given to cyclohexatriene—benzene.
Double Bonds Flip?
Since it would be possible to produce three double bonds by elimination of some atoms of a cyclohexane derivative, it becomes obvious that as quickly as the three bonds appear, they change. They do not flip back and forth between 1,3,5-cyclohexatriene double bonds and 2,4,6-cyclohexatriene double bonds.
From this, it is evident it’s a matter of electron density between the carbon pairs, and not artificially drawn single dashes and double dashes that reflects reality. When bond locations change, electron density changes.
Does this mean there is absolutely no rigidity to a chemical bond? Or that there is nothing resembling bond flipping during a chemical reaction? In fact, while bonds are not absolutely rigid, nor do they flip, molecules do exhibit characteristics not totally dissimilar.
Note: You might also enjoy Mysterious St. Elmo’s Fire
References:
← Back to Classic Science
← Home
