 Does Ice Cool Warm Water? Your tap water is too warm to enjoy, so you run it over a glassful of ice. Then you stop and think: did the ice cool down the warm water? Technically speaking, the answer is a resounding NO! Rather, energy in the warm water heated the ice. Ice is water that lacks sufficient heat energy to melt.
Does Ice Cool Warm Water? Your tap water is too warm to enjoy, so you run it over a glassful of ice. Then you stop and think: did the ice cool down the warm water? Technically speaking, the answer is a resounding NO! Rather, energy in the warm water heated the ice. Ice is water that lacks sufficient heat energy to melt.
Matrix
The mechanism of melting requires the breakdown of a rigid matrix, converting the solid ice into free-flowing, liquid water. That phenomenon doesn’t elevate temperature. Once enough energy has been supplied to break down the matrix, whatever heat is added after that raises the temperature.
So How Does Ice Cool Warm Water?
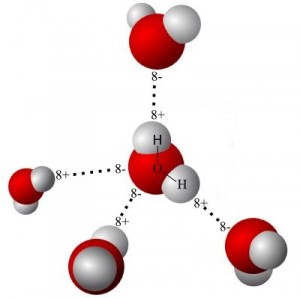
The result of pouring warm water over cold ice is the melting of the outermost layers of ice making up the cube. Water surrounding the ice bridges to the outer surface of the ice via hydrogen bonds. These provide and transfer energy.
In effect, the hydrogen bonds are like little ropes that pull on the outer water molecules. In addition to imparting kinetic energy in the form of motion, these “ropes” enable those molecules to escape from the surface. As one layer is depleted, the process repeats. Energy from the warm water is now reduced. It now makes a cool and refreshing drink.
Note: You might also enjoy Tongue Stuck to a Silver Spoon Eating Ice Cream?
References:

Can you [provide] a better definition of what happens while it is melting?
Although the article was intended to give a quick answer to the question, I agreed with your request to flesh out the explanation a bit, Tristan.
How fast does ice cool a cup of hot water?
It doesn’t. The hot water melts the ice.