The water molecule, H₂O, consists of two hydrogen atoms and one oxygen atom. A naïve attempt at writing its structure out in full is H‒O‒H. What’s naïve about this? This drawing is linear—a straight line. It is naïve because the water molecule is a bent water molecule… bent at about 104.5°.
It is a good thing for us that this is so, since this imparts a degree of polarity to the water molecule. Polarity, in turn, gives rise to hydrogen bonding. The hydrogen bonding of molecules assures water’s liquidity.
In addition hydrogen bonding influences water’s crystallization, so that ice is lighter than very cold water. Ice thus floats, forming an insulating blanket atop lakes and other bodies of water. This prevents them from freezing solid, killing all life within those bodies.
But a Question Arises
All well and good. But the inescapable question arises, “Why is it a bent water molecule and not straight?” What physical and/or chemical principles cause what would seem should be a straight molecule to bend? The answer lies with oxygen’s unbonded electron pairs. What is an unbonded pair?
Unbonded Electron Pairs
The oxygen atom center or nucleus ordinarily contains eight protons plus eight neutrons. A proton has a charge of +1 and a neutron of 0. To balance this electrical charge, the oxygen atom also possesses eight negative electrons in orbitals surrounding the nucleus.
Quantum enthusiasts will label the electronic structure: 1s² 2s² 2p⁴. Shell one contains only an “s” subshell, so it is completely filled. Shell two is not completely filled. The “p” subshell can hold 6 electrons, not just 4. The 2s + 4p electrons are called valence electrons. Oxygen has 6 valence electrons.
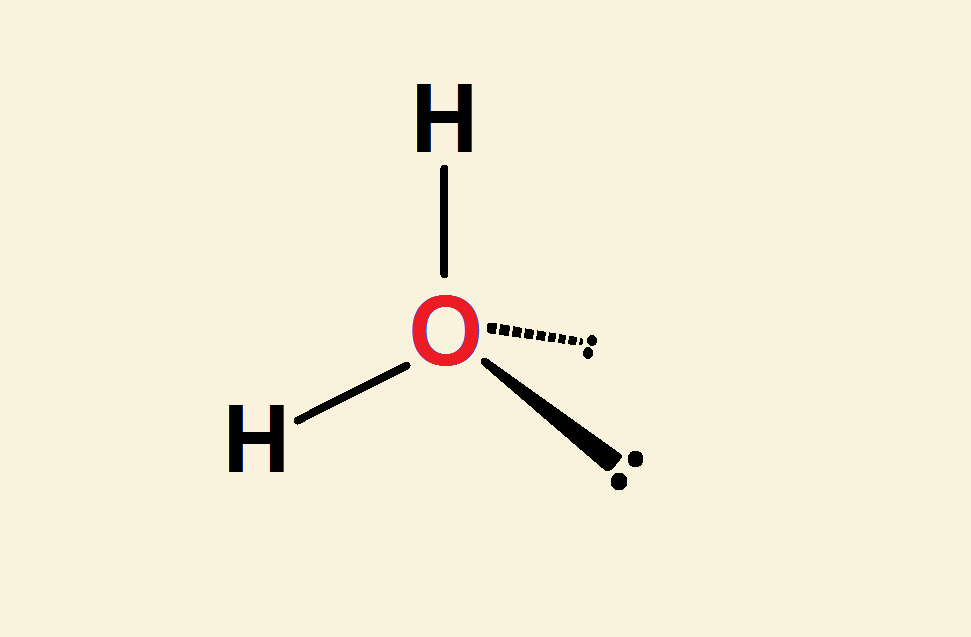
Each hydrogen atom contributes just one electron, to complete the octet, 2s² 2p⁶. The other 4 valence electrons do not bond to hydrogen. They are unbonded. Quantum mechanics dictates they associate in pairs.
The Bent Water Molecule
In view of the above, it becomes apparent the structure of water is much more accurately represented as an oxygen atom attached to two hydrogen atoms and two electron pairs. Notice the essentially tetrahedral layout in the image.
Note: You might also enjoy The Dipole Molecule Water – Mickey the Dipole
References:

It’s a long way from the valency theory I learned over 50 years ago!
Your line “Quantum mechanics dictates they associate in pairs” seems to be the key factor.
I am always left wondering why the bonded-pairs are not opposite each other, and the unbonded pairs opposite each other – symetrical and planar – everybody else seems to ignore this possibility without any explanation. Thank You.
I write articles and answer simple questions about the material, but cannot take time to answer more complex questions. However, this article I also published may help. https://www.quirkyscience.com/hybrid-atomic-orbitals/ Also, this article may be interesting to you in connection with the molecular bend… https://www.quirkyscience.com/what-makes-ice-slippery/