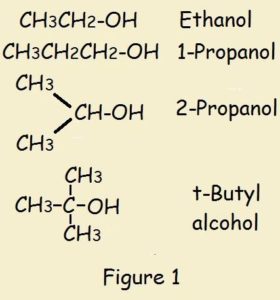
What is alcohol protonation? An alcohol is an organic compound. Some examples are ethanol (ethyl alcohol), isopropyl alcohol, and t-butyl alcohol.
They have the generic chemical formula ROH. H is hydrogen, O is oxygen, and R is any aliphatic (carbon chain) group.
Alcohols are important organic synthetic reagents. During a reaction process, alcohols may be protonated by mineral acids.¹ Alcohol protonation is the adding of a proton. Ethanol, for instance, protonates accordingly,
C₂H₅-OH + H⁺ → C₂H₅-OH₂⁺
Notice from the protonated structure that the final three atoms closely resemble water (H₂O). Water is quite stable. Thus, it is a “good leaving group.” What remains is a carbocation. It should be noted is the charge is now not on the oxygen atom. It is on a carbon atom. The process is written,
C₂H₅-OH₂⁺ → C₂H₅⁺ + H₂O
The “Problem” of Rearrangement
Ever heard the saying, “Every cloud has a silver lining?” Well, molecular rearrangement is our cloud. But in this case, a cloud can be mined for silver. Here’s an example of rearrangement:
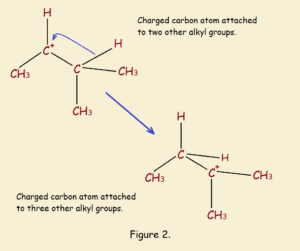
CH₃CH⁺CH(CH₃)₂ → CH₃CH₂C⁺(CH₃)₂
Very common in carbocation rearrangements are 1,2-hydride shifts, such as that shown above and in Figure 2. There are also 1,2-alkyl shifts. Still more rearrangement shifts can occur.
 Additional Rearrangement Shifts
Additional Rearrangement Shifts
Other rearrangement shifts occur, such as the sigmatropic shifts. Note the 1,5-sigmatropic shift illustrated by the University of Liverpool for a methyl derivative of cyclopentadiene…
Alcohol Protonation: Of What Use?
Of what use are intermediates from alcohol protonation? From them we can derive esters, ethers, alkenes, halides, and other useful end products. In fact, the alcohol may not be of much importance. Rather, a simple, short chain alcohol can lead to the developing of larger, more complex species.
¹ Mineral acids include hydrochloric, hydrobromic, and sulfuric.
Note: You might also enjoy Keto Enol Tautomerism
References:
- UC – Davis: Carbocation Rearrangements
- University of Memphis: Carbocation Rearrangement
- ACS Publications: JOC: Lewis acid catalyzed 1,2 aryl shift…

Which has [a greater] tendency to protonate – 1 deg or 2 deg alcohols?
Secondary (2 deg). See if this article provides insight https://www.quirkyscience.com/silicon-tetrachloride-acts-like-strongly-electronegative-atom/